Many drug candidates are poorly water-soluble. Microenvironmental pH (pHM) modification in buccal/sublingual dosage forms has attracted increasing interest as a promising pharmaceutical strategy to enhance the oral mucosal absorption of drugs with pH-dependent solubility. Optimizing drug absorption at the oral mucosa using pHM modification is considered to be a compromise between drug solubility and drug lipophilicity (Log D)/permeation. To create a desired pHM around formulations during the dissolution process, a suitable amount of pH modifiers should be added in the formulations, and the appropriate methods of pHM measurement are required.
- microenvironmental pH modification
- buccal/sublingual dosage form
- solubility
1. Introduction
2. Concept of Microenvironmental pH (pHM) Modification in the Buccal/Sublingual Dosage Forms
2.1. Theory: pH-Dependent Dissolution and Permeation
Drug dissolution/release from buccal/sublingual formulations is one of the crucial factors affecting drug absorption at the oral mucosa. The relationship between the pH, drug solubility and dissolution rate has been elucidated using the Nernst-Noyes-Whitney equation [18] (Equation (1)) and the “solubility-pH” equations (take monoacidic drugs and monobasic drugs as examples) [19,20][19][20] (Equations (2) and (3)), as described below: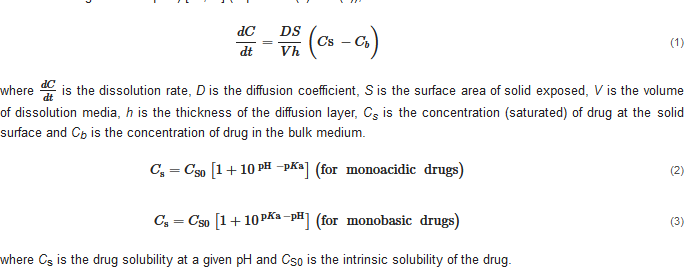 where is the dissolution rate, D is the diffusion coefficient, S is the surface area of solid exposed, V is the volume of dissolution media, h is the thickness of the diffusion layer, Cs is the concentration (saturated) of drug at the solid surface and Cb is the concentration of drug in the bulk medium.
where Cs is the drug solubility at a given pH and CS0 is the intrinsic solubility of the drug.
According to the “Solubility-pH” equations, a slight shift in the pH might lead to a significant change in the drug solubility. Theoretically, decreasing the pH could improve the solubility of a weakly basic drug by increasing the concentration of ionized drug in the solution. When most of the dissolved drug substance remains in its ionized form, a further decrease in the pH has little effect on its solubility, and the drug solubility approaches a plateau level in the pH-solubility profile. A suitable pH level at the surface of a solid formulation exposed to dissolution media could increase the local drug concentration (Cs) and, consequently, enhance the drug dissolution and release (dCdt
) from the solid formulation.
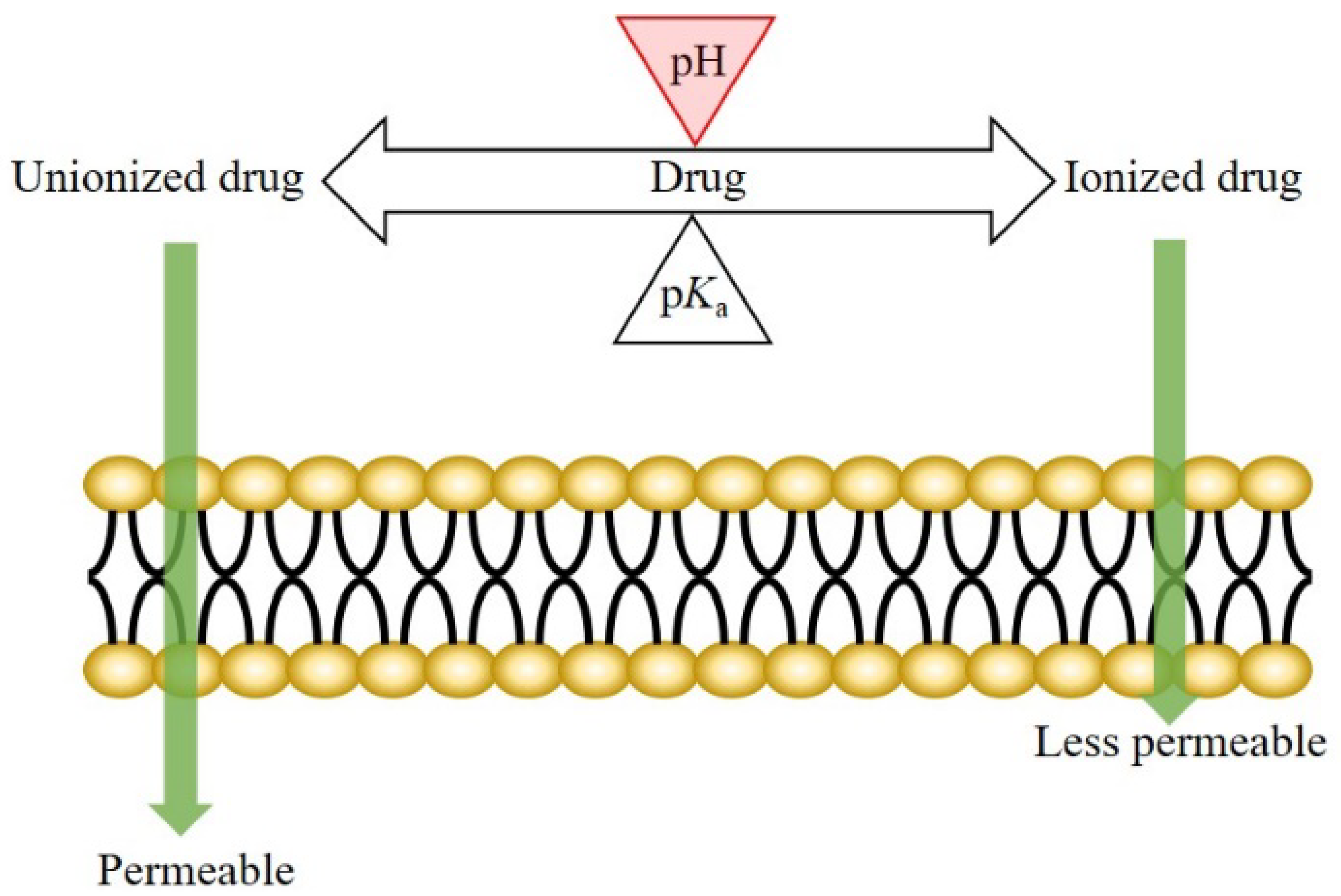
The mechanism of drug transport across the oral epithelium is similar to that across the other epithelia in the human body. Generally, both the transcellular and paracellular pathways are involved in this process [3,21,22,23,24][3][21][22][23][24]. For the drugs transported mainly via the transcellular route, drug permeation across the oral mucosa might be affected by the pH at the oral mucosa. According to the pH-partition theory, the neutral forms of drugs are more permeable (lipophilic) than the ionized species; therefore, a pH shift not only affects the dissociation of weakly ionizable drugs, but also the drug permeation across biological membranes (Figure 1) [25,26][25][26].
where is the dissolution rate, D is the diffusion coefficient, S is the surface area of solid exposed, V is the volume of dissolution media, h is the thickness of the diffusion layer, Cs is the concentration (saturated) of drug at the solid surface and Cb is the concentration of drug in the bulk medium.
where Cs is the drug solubility at a given pH and CS0 is the intrinsic solubility of the drug.
According to the “Solubility-pH” equations, a slight shift in the pH might lead to a significant change in the drug solubility. Theoretically, decreasing the pH could improve the solubility of a weakly basic drug by increasing the concentration of ionized drug in the solution. When most of the dissolved drug substance remains in its ionized form, a further decrease in the pH has little effect on its solubility, and the drug solubility approaches a plateau level in the pH-solubility profile. A suitable pH level at the surface of a solid formulation exposed to dissolution media could increase the local drug concentration (Cs) and, consequently, enhance the drug dissolution and release (dCdt
) from the solid formulation.
The mechanism of drug transport across the oral epithelium is similar to that across the other epithelia in the human body. Generally, both the transcellular and paracellular pathways are involved in this process [3,21,22,23,24][3][21][22][23][24]. For the drugs transported mainly via the transcellular route, drug permeation across the oral mucosa might be affected by the pH at the oral mucosa. According to the pH-partition theory, the neutral forms of drugs are more permeable (lipophilic) than the ionized species; therefore, a pH shift not only affects the dissociation of weakly ionizable drugs, but also the drug permeation across biological membranes (Figure 1) [25,26][25][26].
2.2. pH
max
Concept
2.3. Microenvironmental pH Modification in Buccal/Sublingual Dosage Forms
2.3. Microenvironmental pH Modification in Buccal/Sublingual Dosage Forms
3. Properties of Saliva Associated with pH Modification
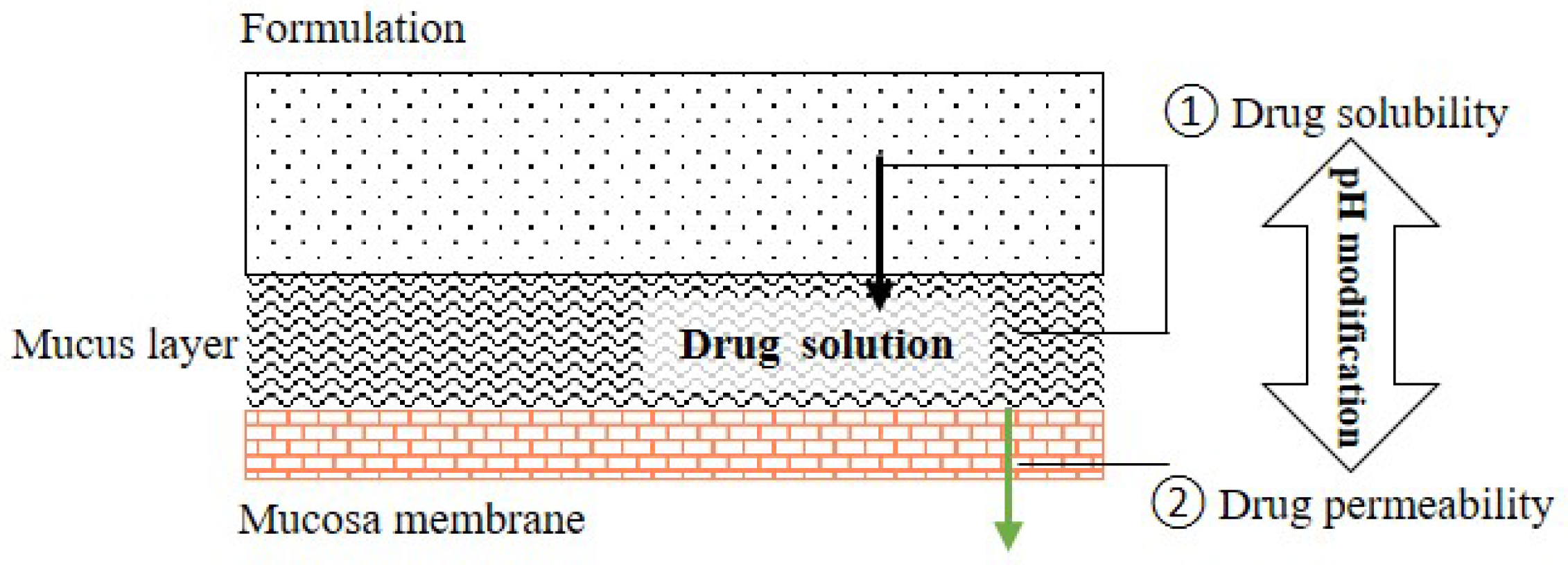
The main functions of saliva are to maintain oral health and help to build and maintain the health of hard and soft tissues. Approximately 99% of saliva is water, and the other 1% consists of a variety of electrolytes and proteins [30,31]. Regarding buccal/sublingual drug delivery, saliva provides a water-rich environment that facilitates in the drug dissolution and release from buccal/sublingual formulations before the drugs permeate through the membrane of oral mucosa [32]. To achieve a successful pH
The main functions of saliva are to maintain oral health and help to build and maintain the health of hard and soft tissues. Approximately 99% of saliva is water, and the other 1% consists of a variety of electrolytes and proteins [30][31]. Regarding buccal/sublingual drug delivery, saliva provides a water-rich environment that facilitates in the drug dissolution and release from buccal/sublingual formulations before the drugs permeate through the membrane of oral mucosa [32]. To achieve a successful pH
M
modification in buccal/sublingual formulations, some properties of saliva should be taken into consideration during the formulation design.
3.1. pH and Buffer Capacity of Saliva
3.2. Secretion Rate of Saliva and Thickness of Salivary Film
3.2. Secretion Rate of Saliva and Thickness of Salivary Film
4. Drug Candidate and pH Modifier for Buccal/Sublingual Dosage Forms
4.1. Drug Candidate
The low drug loading capacity of buccal/sublingual formulations and the limited absorption area in the oral cavity are two main limitations for buccal/sublingual drug delivery. Thus, drug candidates should be high potency to achieve successful therapeutic efficacy. In addition, suitable drug candidates must not cause local irritation and toxicity at oral mucosa. Regarding physicochemical properties, high lipophilicity (log P (octanol/water) > 2), fairly good water-solubility and small molecular size (less than 800 Da) are typically considered as ideal parameters for drug candidates, as described previously [3]. The extent of different drug transport pathways across the epithelium depends on the drug physicochemical properties [43,44][39][40]. Typically, drug candidates with high lipophilicity can move across the lipid-rich epithelial cell membrane with relative ease. Fairly good water solubility allows for the fast drug release of buccal/sublingual formulations and drug diffusion across the hydrophilic cytoplasm of cells and paracellular passage. Macromolecules can be delivered via the oral mucosa, e.g., buccal insulin spray (Generex Oral-lyn®) was approved by Food and Drug Administration (FDA) for the treatment of patients under the Investigational New Drug (IND) program [45,46,47][41][42][43]. However, the number of marketed buccal/sublingual macromolecules is very small. However, over 40% of marketed drugs and approximately 90% of drug candidates are reported to be poorly water-soluble [5], and most of them are weakly ionizable drugs, indicating that their solubility and/or permeability across the lipid-rich epithelium are pH-dependent [49,50,51,52][44][45][46][47]. Typically, the ionic form of a drug is more water soluble than its non-ionic form. A change in the pH might influence the ratio of the ionized form of the dissolved drug, according to the Henderson-Hasselbach equation (Equation (4)) [53][48]. When the difference in the water solubility (and/or lipophilicity) between the two forms is big enough, a slight pH change might have a significant effect on the drug solubility. Therefore, drug candidates suitable for pHM modification should have pH-dependent solubility and/or pH-dependent lipophilicity and be poorly soluble at physiological pH in the oral cavity.4.2. pH Modifier
There are a few concerns about the excipients used in pharmaceutical formulations. A pH modifier can only be considered as a pharmaceutical excipient if it has been demonstrated to be safe for human beings. So far, various pH modifiers have been applied in the food and pharmaceutical industries. The Generally Recognized as Safe (GRAS) list of the FDA lists some safe pH modifiers that have been added to food. In addition, various pH modifiers recommended for oral liquids have been collected in the United States Pharmacopeia (USP). However, the specific pH modifiers for buccal/sublingual formulations were not referenced. The pH modifiers collected in the USP [67][49] and their maximum potency per unit dose used in solid oral and buccal/sublingual formulations in the database of Inactive Ingredient Search for Approved Drug Products Search, provided by the FDA [68][50]. The pH modifiers can be divided into three categories: acidifying agents, alkalizing agents and buffering agents. Currently, only a few pH modifiers, were applied in the commercial buccal/sublingual formulations approved by the FDA. pH modifiers demonstrated without local irritation and toxicity to oral mucosa could also be potential choices for the buccal/sublingual dosage forms.5. Methods for Microenvironmental pH Measurement
5.1. pH Electrode Approach
The most common method to determine the pHM is the pH electrode approach. As the previous studies described [57[51][52][53][54][55][56][57],77,78,79,80,81,82], formulations (e.g., tablets, film and patch) were allowed to swell in a limited volume of buffer solution (at neutral pH) at room temperature for a certain period. Subsequently, the pH on the surface of the formulations was determined using a pH electrode. Mucoadhesive buccal films containing ornidazole were allowed to swell in 4 mL of phosphate buffer (pH 6.8 ± 0.1) at room temperature for 120 min and the surface pH was measured using an electrode pH meter [77][52].5.2. Computer-Enhanced Color Images Method
To gain more information on the pHM change during the dissolving process of the fentanyl tablet, a computer-enhanced color images (of pH paper) method was used to record the pHM as it varied over the surface of the swelling tablet [54][58]. The schematic view of the setup and the computer-enhanced color images of pH paper are shown in Figure 3. A piece of pH paper was placed over a tablet. The tablet with the pH paper was held between two microscope slides, and a small volume of deionized water was applied to the pH paper. The tablet was rapidly wetted by the water that permeated the pH paper. As the tablet swelled, the pH paper was digitally photographed at different time intervals. The pH over the distinct regions of the tablet surface were then determined from the digital images and in comparison to the reference pH standards. The pHM decreased from 7.0 to 5.0, and then gradually increased to around 6.0 during the first 5 min of the dissolving process [54][58].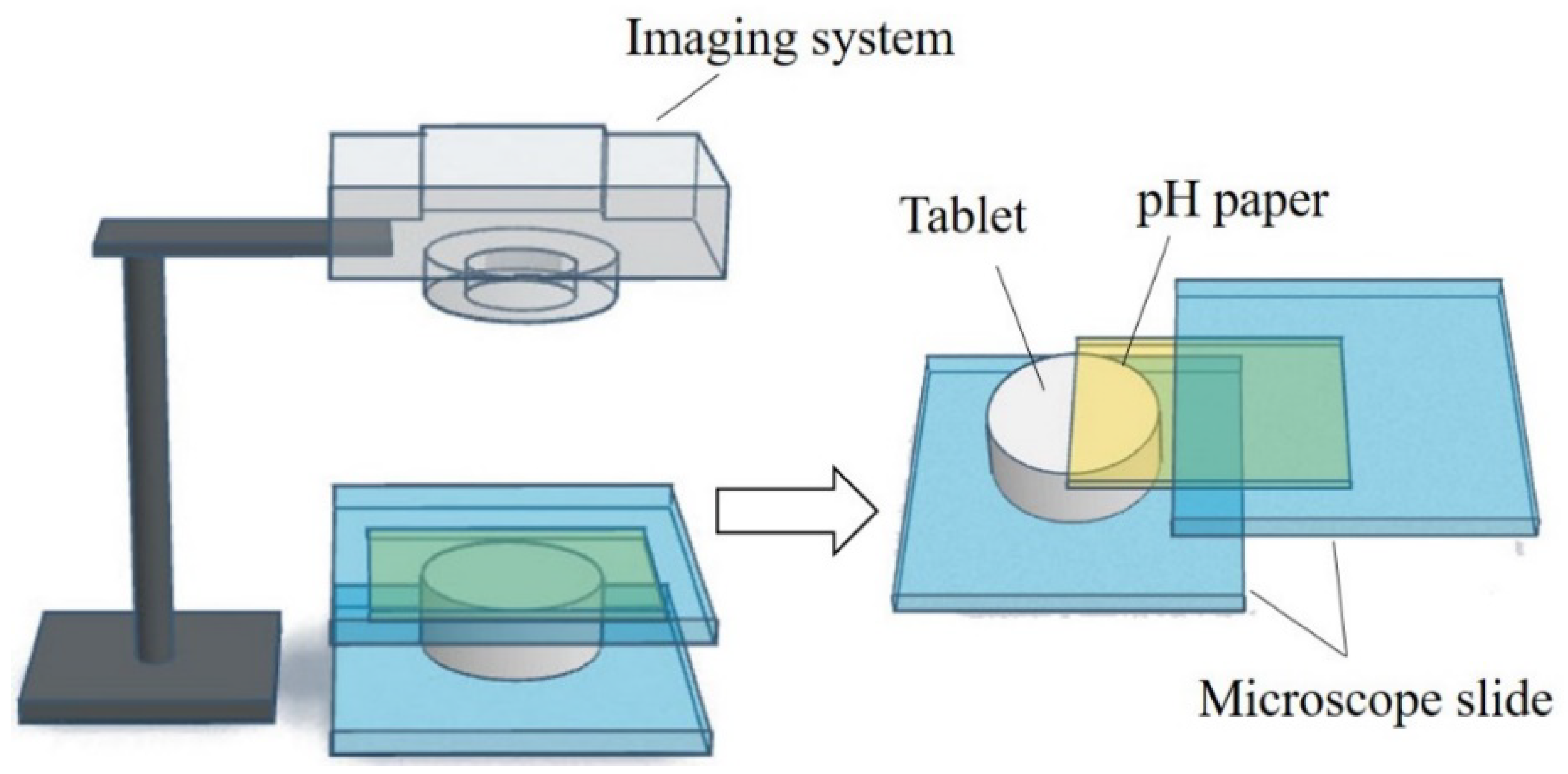
5.3. UV/Vis Imaging Method
6. Microenvironmental pH (pHM) Modification Methods
6.1. Microenvironmental pH Modification Using Acidifying/Alkalizing Agents
6.2. Microenvironmental pH Modification Using Buffering Agents
The pHM in the vicinity of formulations at the oral mucosa is generally affected by the release of the ingredients (particularly the acidic and basic ingredients) from the formulations. The pHM changes over time, along with the ingredients released upon dissolution. To maintain the suitable pHM and achieve optimal drug absorption at the oral mucosa, buffer agents are incorporated in formulations. The addition of buffering agents can form a buffer system in and around the matrix of the formulations and prevent the pHM from changing. This method was demonstrated to be effective in some cases. Phosphate buffer and borate buffer were used in methylcellulose-based gels to create pHM 7.4, 8.5, 9.0 and 9.5 for the buccal delivery of metoprolol in Göttingen minipigs in a previous study. A higher buccal absorption of metoprolol from the gels was observed at higher pH values, and the absolute bioavailability of metoprolol via buccal dosing was significantly higher compared to that via oral administration [29]. In the study, the metoprolol release from the gels might be similar, and the pH has little effect on metoprolol release, because the concentration of methylcellulose used in the gels was the same (1%, w/v) and metoprolol had already been dissolved in the gels. Metoprolol permeability across the buccal mucosa is the rate-limit step for the buccal absorption of metoprolol. Furthermore, metoprolol with pKa 9.56 [85][62] has a pH-dependent lipophilicity and permeability in vitro and ex vivo [29,86][29][63]. Thus, the pH has a crucial influence on the buccal absorption of metoprolol incorporated in gels.6.3. Microenvironmental pH Modification Using Effervescence
Formulations with effervescence generally contain an alkaline agent (e.g., sodium carbonate and sodium bicarbonate) and an acid that is capable of inducing the effervescence reaction during the dissolution [87][64]. The carbonic acid produced from the chemical reaction could decrease the pHM and rapidly convert to water and carbon dioxide. The tablet using an effervescence reaction (containing citric acid and bicarbonate) was employed to enhance the absorption of fentanyl at the buccal mucosa [54,56,88][58][65][66]. A dynamic shift in the pHM (pH was decreased and subsequently be increased) occurred in the microenvironment between the tablet and the buccal mucosa, and the pHM shift might be the main factor for the enhanced buccal absorption of fentanyl. The initial decrease in the pH, caused by the carbonic acid and release of citric acid from the tablet, facilitated the release of fentanyl from the tablet. The pH subsequently increased due to the dissociation of carbonic acid (into CO2 and water) and the dissipation of the CO2, which favored the formation of unionized fentanyl. The unionized fentanyl can move across the lipid-rich oral mucosal membrane with greater ease than the ionized fentanyl [54,56,88][58][65][66].References
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and Revisited Approaches in Nanoparticle Systems for Buccal Drug Delivery. J. Control. Release 2020, 320, 125–141.
- Madhav, N.V.S.; Shakya, A.K.; Shakya, P.; Singh, K. Orotransmucosal Drug Delivery Systems: A Review. J. Control. Release 2009, 140, 2–11.
- Lam, J.K.W.; Xu, Y.; Worsley, A.; Wong, I.C.K. Oral Transmucosal Drug Delivery for Pediatric Use. Adv. Drug Deliv. Rev. 2014, 73, 50–62.
- Zhang, H.; Zhang, J.; Streisand, J.B. Oral Mucosal Drug Delivery. Clin. Pharmacokinet. 2002, 41, 661–680.
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins: Basic Science and Product Development. J. Pharm. Pharmacol. 2010, 62, 1607–1621.
- Taniguchi, C.; Kawabata, Y.; Wada, K.; Yamada, S.; Onoue, S. Microenvironmental PH-Modification to Improve Dissolution Behavior and Oral Absorption for Drugs with PH-Dependent Solubility. Expert Opin. Drug Deliv. 2014, 11, 505–516.
- Yang, M.; He, S.; Fan, Y.; Wang, Y.; Ge, Z.; Shan, L.; Gong, W.; Huang, X.; Tong, Y.; Gao, C. Microenvironmental PH-Modified Solid Dispersions to Enhance the Dissolution and Bioavailability of Poorly Water-Soluble Weakly Basic GT0918, a Developing Anti-Prostate Cancer Drug: Preparation, Characterization and Evaluation in Vivo. Int. J. Pharm. 2014, 475, 97–109.
- Badawy, S.I.F.; Hussain, M.A. Microenvironmental PH Modulation in Solid Dosage Forms. J. Pharm. Sci. 2007, 96, 948–959.
- Doherty, C.; York, P. Microenvironmental PH Control of Drug Dissolution. Int. J. Pharm. 1989, 50, 223–232.
- Younes, N.F.; El Assasy, A.E.-H.I.; Makhlouf, A.I.A. Microenvironmental PH-Modified Amisulpride-Labrasol Matrix Tablets: Development, Optimization and in Vivo Pharmacokinetic Study. Drug Deliv. Transl. Res. 2020, 11, 103–117.
- Onoue, S.; Inoue, R.; Taniguchi, C.; Kawabata, Y.; Yamashita, K.; Wada, K.; Yamauchi, Y.; Yamada, S. Improved Dissolution and Pharmacokinetic Behavior of Dipyridamole Formulation with Microenvironmental PH-Modifier under Hypochlorhydria. Int. J. Pharm. 2012, 426, 61–66.
- Mitra, A.; Kesisoglou, F.; Beauchamp, M.; Zhu, W.; Chiti, F.; Wu, Y. Using Absorption Simulation and Gastric PH Modulated Dog Model for Formulation Development To Overcome Achlorhydria Effect. Mol. Pharm. 2011, 8, 2216–2223.
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of PH and Temperature Profiles in the GI Tract of Fasted Human Subjects Using the Intellicap® System. J. Pharm. Sci. 2015, 104, 2855–2863.
- Baliga, S.; Muglikar, S.; Kale, R. Salivary PH: A Diagnostic Biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465.
- Ciolino, L.A.; McCauley, H.A.; Fraser, D.B.; Wolnik, K.A. The Relative Buffering Capacities of Saliva and Moist Snuff: Implications for Nicotine Absorption. J. Anal. Toxicol. 2001, 25, 15–25.
- Proctor, G.B. The Physiology of Salivary Secretion. Periodontology 2000 2016, 70, 11–25.
- Dawes, C. Physiological Factors Affecting Salivary Flow Rate, Oral Sugar Clearance, and the Sensation of Dry Mouth in Man. J. Dent. Res. 1987, 66, 648–653.
- Dokoumetzidis, A.; Macheras, P. A Century of Dissolution Research: From Noyes and Whitney to the Biopharmaceutics Classification System. Int. J. Pharm. 2006, 321, 1–11.
- Avdeef, A. Solubility of Sparingly-Soluble Ionizable Drugs. Adv. Drug Deliv. Rev. 2007, 59, 568–590.
- Bassi, P.; Kaur, G. PH Modulation: A Mechanism to Obtain PH-Independent Drug Release. Expert Opin. Drug Deliv. 2010, 7, 845–857.
- Pather, S.I.; Rathbone, M.J.; Şenel, S. Current Status and the Future of Buccal Drug Delivery Systems. Expert Opin. Drug Deliv. 2008, 5, 531–542.
- Mashru, R.; Sutariya, V.; Sankalia, M.; Sankalia, J. Transbuccal Delivery of Lamotrigine across Porcine Buccal Mucosa: In Vitro Determination of Routes of Buccal Transport. J. Pharm. Pharm. Sci. 2005, 8, 54–62.
- Birudaraj, R.a.j.; Berner, B.; Shen, S.; Li, X. Buccal Permeation of Buspirone: Mechanistic Studies on Transport Pathways. J. Pharm. Sci. 2005, 94, 70–78.
- Nielsen, H.M.; Rassing, M.R. Nicotine Permeability across the Buccal TR146 Cell Culture Model and Porcine Buccal Mucosa in Vitro: Effect of PH and Concentration. Eur. J. Pharm. Sci. 2002, 16, 151–157.
- Thomae, A.V.; Wunderli-Allenspach, H.; Krämer, S.D. Permeation of Aromatic Carboxylic Acids across Lipid Bilayers: The PH-Partition Hypothesis Revisited. Biophys. J. 2005, 89, 1802–1811.
- Shore, P.A.; Brodie, B.B.; Hogben, C.A.M. The Gastric Secretion of Drugs: A Ph Partition Hypothesis. J. Pharmacol. Exp. Ther. 1957, 119, 361–369.
- Wang, Y.; Zuo, Z.; Chen, X.; Tomlinson, B.; Chow, M.S.S. Improving Sublingual Delivery of Weak Base Compounds Using PHmax Concept: Application to Propranolol. Eur. J. Pharm. Sci. 2010, 39, 272–278.
- Meng-Lund, E.; Jacobsen, J.; Andersen, M.B.; Jespersen, M.L.; Karlsson, J.-J.; Garmer, M.; Jørgensen, E.B.; Holm, R. Conscious and Anaesthetised Göttingen Mini-Pigs as an in-Vivo Model for Buccal Absorption—PH-Dependent Absorption of Metoprolol from Bioadhesive Tablets. Drug Dev. Ind. Pharm. 2014, 40, 604–610.
- Holm, R.; Meng-Lund, E.; Andersen, M.B.; Jespersen, M.L.; Karlsson, J.-J.; Garmer, M.; Jørgensen, E.B.; Jacobsen, J. In Vitro, Ex Vivo and in Vivo Examination of Buccal Absorption of Metoprolol with Varying PH in TR146 Cell Culture, Porcine Buccal Mucosa and Göttingen Minipigs. Eur. J. Pharm. Sci. 2013, 49, 117–124.
- Dodds, M.; Roland, S.; Edgar, M.; Thornhill, M. Saliva A Review of Its Role in Maintaining Oral Health and Preventing Dental Disease. BDJ Team 2015, 2, 15123.
- de Almeida, P.D.V.; Grégio, A.M.T.; Machado, M.A.; de Lima, A.A.S.; Azevedo, L.R. Saliva Composition and Functions: A Comprehensive Review. J. Contemp. Dent. Pract. 2008, 9, 72–80.
- Patel, V.F.; Liu, F.; Brown, M.B. Advances in Oral Transmucosal Drug Delivery. J. Control. Release 2011, 153, 106–116.
- Aframian, D.; Davidowitz, T.; Benoliel, R. The Distribution of Oral Mucosal PH Values in Healthy Saliva Secretors. Oral Dis. 2006, 12, 420–423.
- Tenovuo, J. Salivary Parameters of Relevance for Assessing Caries Activity in Individuals and Populations. Community Dent. Oral Epidemiol. 1997, 25, 82–86.
- Lazarchik, D.A.; Filler, S.J. Effects of Gastroesophageal Reflux on the Oral Cavity. Am. J. Med. 1997, 103, 107S–113S.
- Bardow, A.; Moe, D.; Nyvad, B.; Nauntofte, B. The Buffer Capacity and Buffer Systems of Human Whole Saliva Measured without Loss of CO2. Arch. Oral Biol. 2000, 45, 1–12.
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary Secretion in Health and Disease. J. Oral Rehabil. 2018, 45, 730–746.
- Pijpe, J.; Kalk, W.W.I.; Bootsma, H.; Spijkervet, F.K.L.; Kallenberg, C.G.M.; Vissink, A. Progression of Salivary Gland Dysfunction in Patients with Sjögren’s Syndrome. Ann. Rheum. Dis. 2007, 66, 107–112.
- Xiang, J.; Fang, X.; Li, X. Transbuccal Delivery of 2′,3′-Dideoxycytidine: In Vitro Permeation Study and Histological Investigation. Int. J. Pharm. 2002, 231, 57–66.
- Deneer, V.H.M.; Drese, G.B.; Roemelé, P.E.H.; Verhoef, J.C.; Lie-A-Huen, L.; Kingma, J.H.; Brouwers, J.R.B.J.; Junginger, H.E. Buccal Transport of Flecainide and Sotalol: Effect of a Bile Salt and Ionization State. Int. J. Pharm. 2002, 241, 127–134.
- Easa, N.; Alany, R.G.; Carew, M.; Vangala, A. A Review of Non-Invasive Insulin Delivery Systems for Diabetes Therapy in Clinical Trials over the Past Decade. Drug Discov. Today 2019, 24, 440–451.
- Morales, J.O.; Brayden, D.J. Buccal Delivery of Small Molecules and Biologics: Of Mucoadhesive Polymers, Films, and Nanoparticles. Curr. Opin. Pharmacol. 2017, 36, 22–28.
- Heinemann, L.; Jacques, Y. Oral Insulin and Buccal Insulin: A Critical Reappraisal. J. Diabetes Sci. Technol. 2009, 3, 568–584.
- Zamora, W.J.; Curutchet, C.; Campanera, J.M.; Luque, F.J. Prediction of PH-Dependent Hydrophobic Profiles of Small Molecules from Miertus–Scrocco–Tomasi Continuum Solvation Calculations. J. Phys. Chem. B 2017, 121, 9868–9880.
- Zamora, W.J.; Campanera, J.M.; Luque, F.J. Development of a Structure-Based, PH-Dependent Lipophilicity Scale of Amino Acids from Continuum Solvation Calculations. J. Phys. Chem. Lett. 2019, 10, 883–889.
- Xing, L.; Glen, R.C. Novel Methods for the Prediction of LogP, PKa, and LogD. J. Chem. Inf. Comput. Sci. 2002, 42, 796–805.
- Iwanaga, K.; Kato, S.; Miyazaki, M.; Kakemi, M. Enhancing the Intestinal Absorption of Poorly Water-Soluble Weak-Acidic Compound by Controlling Local PH. Drug Dev. Ind. Pharm. 2013, 39, 1887–1894.
- Henderson—Hasselbalch Equation. In Manual of Pharmacologic Calculations: With Computer Programs; Tallarida, R.J.; Murray, R.B. (Eds.) Springer: New York, NY, USA, 1987; pp. 74–75. ISBN 978-1-4612-4974-0.
- The United States. Pharmacopeial Convention Excipients: USP and NF Excipients, Listed by Functional Category. In United States Pharmacopeia and National Formulary; The United States Pharmacopeial Convention: North Bethesda, MD, USA, 2016; p. 7490.
- Food and Drug Administration. FDA-Inactive Ingredient Search for Approved Drug Products Search. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm (accessed on 1 October 2022).
- Elagamy, H.I.; Essa, E.A.; Nouh, A.; El Maghraby, G.M. Development and Evaluation of Rapidly Dissolving Buccal Films of Naftopidil: In Vitro and in Vivo Evaluation. Drug Dev. Ind. Pharm. 2019, 45, 1695–1706.
- Zhang, C.; Liu, Y.; Li, W.; Gao, P.; Xiang, D.; Ren, X.; Liu, D. Mucoadhesive Buccal Film Containing Ornidazole and Dexamethasone for Oral Ulcers: In Vitro and in Vivo Studies. Pharm. Dev. Technol. 2019, 24, 118–126.
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive Delivery Systems. I. Evaluation of Mucoadhesive Polymers for Buccal Tablet Formulation. Drug Dev. Ind. Pharm. 2004, 30, 985–993.
- Patel, V.M.; Prajapati, B.G.; Patel, H.V.; Patel, K.M. Mucoadhesive Bilayer Tablets of Propranolol Hydrochloride. AAPS PharmSciTech 2007, 8, E203–E208.
- Mohamad, S.A.; Abdelkader, H.; Elrehany, M.; Mansour, H.F. Vitamin B12 Buccoadhesive Tablets: Auspicious Non-Invasive Substitute for Intra Muscular Injection: Formulation, in Vitro and in Vivo Appraisal. Drug Dev. Ind. Pharm. 2019, 45, 244–251.
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive Buccal Patches of Miconazole Nitrate: In Vitro/in Vivo Performance and Effect of Ageing. Int. J. Pharm. 2003, 264, 1–14.
- Chen, G.; Bunt, C.; Wen, J. Mucoadhesive Polymers-Based Film as a Carrier System for Sublingual Delivery of Glutathione. J. Pharm. Pharmacol. 2015, 67, 26–34.
- Durfee, S.; Messina, J.; Khankari, R. Fentanyl Effervescent Buccal Tablets. Am. J. Drug Deliv. 2006, 4, 1–5.
- He, S.; Østergaard, J.; Ashna, M.; Nielsen, C.U.; Jacobsen, J.; Mu, H. Microenvironmental PH Modifying Films for Buccal Delivery of Saquinavir: Effects of Organic Acids on PH and Drug Release in Vitro. Int. J. Pharm. 2020, 585, 119567.
- Fouad, S.A.; Shamma, R.N.; Basalious, E.B.; El-Nabarawi, M.A.; Tayel, S.A. Novel Instantly-Soluble Transmucosal Matrix (ISTM) Using Dual Mechanism Solubilizer for Sublingual and Nasal Delivery of Dapoxetine Hydrochloride: In-Vitro/in-Vivo Evaluation. Int. J. Pharm. 2016, 505, 212–222.
- Aldawsari, H.M.; Badr-Eldin, S.M. Enhanced Pharmacokinetic Performance of Dapoxetine Hydrochloride via the Formulation of Instantly-Dissolving Buccal Films with Acidic PH Modifier and Hydrophilic Cyclodextrin: Factorial Analysis, in Vitro and in Vivo Assessment. J. Adv. Res. 2020, 24, 281–290.
- Avdeef, A.; Berger, C.M. PH-Metric Solubility.: 3. Dissolution Titration Template Method for Solubility Determination. Eur. J. Pharm. Sci. 2001, 14, 281–291.
- Zur, M.; Gasparini, M.; Wolk, O.; Amidon, G.L.; Dahan, A. The Low/High BCS Permeability Class Boundary: Physicochemical Comparison of Metoprolol and Labetalol. Mol. Pharm. 2014, 11, 1707–1714.
- Advankar, A.; Maheshwari, R.; Tambe, V.; Todke, P.; Raval, N.; Kapoor, D.; Tekade, R.K. Chapter 13—Specialized Tablets: Ancient History to Modern Developments. In Drug Delivery Systems; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 615–664. ISBN 978-0-12-814487-9.
- Darwish, M.; Tempero, K.; Jiang, J.G.; Simonson, P.G. Relative Bioavailability of Fentanyl Following Various Dosing Regimens of Fentanyl Buccal Tablet in Healthy Japanese Volunteers. Arch. Drug Inf. 2008, 1, 56–62.
- Freye, E. A New Transmucosal Drug Delivery System for Patients with Breakthrough Cancer Pain: The Fentanyl Effervescent Buccal Tablet. Available online: https://www.dovepress.com/a-new-transmucosal-drug-delivery-system-for-patients-with-breakthrough-peer-reviewed-article-JPR (accessed on 30 September 2020).
