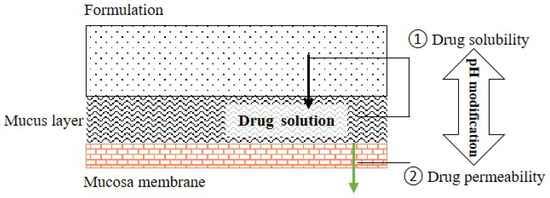
Many drug candidates are poorly water-soluble. Microenvironmental pH (pHM) modification in buccal/sublingual dosage forms has attracted increasing interest as a promising pharmaceutical strategy to enhance the oral mucosal absorption of drugs with pH-dependent solubility. Optimizing drug absorption at the oral mucosa using pHM modification is considered to be a compromise between drug solubility and drug lipophilicity (Log D)/permeation. To create a desired pHM around formulations during the dissolution process, a suitable amount of pH modifiers should be added in the formulations, and the appropriate methods of pHM measurement are required.
- microenvironmental pH modification
- buccal/sublingual dosage form
- solubility
1. Introduction
2. Concept of Microenvironmental pH (pHM) Modification in the Buccal/Sublingual Dosage Forms
2.1. Theory: pH-Dependent Dissolution and Permeation
Drug dissolution/release from buccal/sublingual formulations is one of the crucial factors affecting drug absorption at the oral mucosa. The relationship between the pH, drug solubility and dissolution rate has been elucidated using the Nernst-Noyes-Whitney equation [18] (Equation (1)) and the “solubility-pH” equations (take monoacidic drugs and monobasic drugs as examples) [19][20][19,20] (Equations (2) and (3)), as described below: where is the dissolution rate, D is the diffusion coefficient, S is the surface area of solid exposed, V is the volume of dissolution media, h is the thickness of the diffusion layer, Cs is the concentration (saturated) of drug at the solid surface and Cb is the concentration of drug in the bulk medium.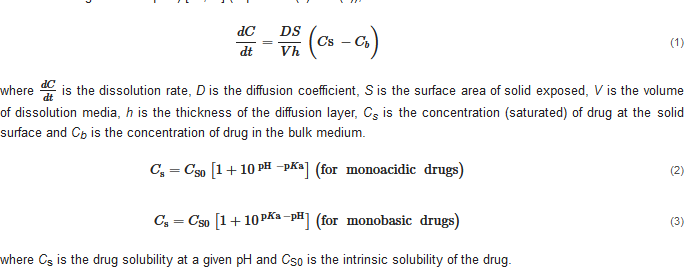 where Cs is the drug solubility at a given pH and CS0 is the intrinsic solubility of the drug.
According to the “Solubility-pH” equations, a slight shift in the pH might lead to a significant change in the drug solubility. Theoretically, decreasing the pH could improve the solubility of a weakly basic drug by increasing the concentration of ionized drug in the solution. When most of the dissolved drug substance remains in its ionized form, a further decrease in the pH has little effect on its solubility, and the drug solubility approaches a plateau level in the pH-solubility profile. A suitable pH level at the surface of a solid formulation exposed to dissolution media could increase the local drug concentration (Cs) and, consequently, enhance the drug dissolution and release (dCdt
) from the solid formulation.
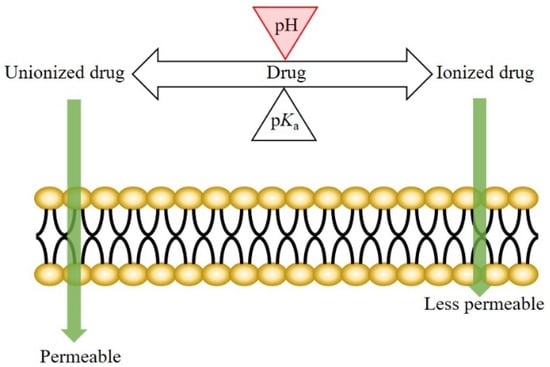
The mechanism of drug transport across the oral epithelium is similar to that across the other epithelia in the human body. Generally, both the transcellular and paracellular pathways are involved in this process [3][21][22][23][24][3,21,22,23,24]. For the drugs transported mainly via the transcellular route, drug permeation across the oral mucosa might be affected by the pH at the oral mucosa. According to the pH-partition theory, the neutral forms of drugs are more permeable (lipophilic) than the ionized species; therefore, a pH shift not only affects the dissociation of weakly ionizable drugs, but also the drug permeation across biological membranes (Figure 1) [25][26][25,26].
where Cs is the drug solubility at a given pH and CS0 is the intrinsic solubility of the drug.
According to the “Solubility-pH” equations, a slight shift in the pH might lead to a significant change in the drug solubility. Theoretically, decreasing the pH could improve the solubility of a weakly basic drug by increasing the concentration of ionized drug in the solution. When most of the dissolved drug substance remains in its ionized form, a further decrease in the pH has little effect on its solubility, and the drug solubility approaches a plateau level in the pH-solubility profile. A suitable pH level at the surface of a solid formulation exposed to dissolution media could increase the local drug concentration (Cs) and, consequently, enhance the drug dissolution and release (dCdt
) from the solid formulation.
The mechanism of drug transport across the oral epithelium is similar to that across the other epithelia in the human body. Generally, both the transcellular and paracellular pathways are involved in this process [3][21][22][23][24][3,21,22,23,24]. For the drugs transported mainly via the transcellular route, drug permeation across the oral mucosa might be affected by the pH at the oral mucosa. According to the pH-partition theory, the neutral forms of drugs are more permeable (lipophilic) than the ionized species; therefore, a pH shift not only affects the dissociation of weakly ionizable drugs, but also the drug permeation across biological membranes (Figure 1) [25][26][25,26].
2.2. pH
max
Concept
2.3. Microenvironmental pH Modification in Buccal/Sublingual Dosage Forms
2.3. Microenvironmental pH Modification in Buccal/Sublingual Dosage Forms
3. Properties of Saliva Associated with pH Modification
The main functions of saliva are to maintain oral health and help to build and maintain the health of hard and soft tissues. Approximately 99% of saliva is water, and the other 1% consists of a variety of electrolytes and proteins [30][31]. Regarding buccal/sublingual drug delivery, saliva provides a water-rich environment that facilitates in the drug dissolution and release from buccal/sublingual formulations before the drugs permeate through the membrane of oral mucosa [32]. To achieve a successful pH
The main functions of saliva are to maintain oral health and help to build and maintain the health of hard and soft tissues. Approximately 99% of saliva is water, and the other 1% consists of a variety of electrolytes and proteins [30,31]. Regarding buccal/sublingual drug delivery, saliva provides a water-rich environment that facilitates in the drug dissolution and release from buccal/sublingual formulations before the drugs permeate through the membrane of oral mucosa [32]. To achieve a successful pH
M
modification in buccal/sublingual formulations, some properties of saliva should be taken into consideration during the formulation design.
3.1. pH and Buffer Capacity of Saliva
3.2. Secretion Rate of Saliva and Thickness of Salivary Film
3.2. Secretion Rate of Saliva and Thickness of Salivary Film
Saliva is a complex mixture secreted by salivary glands. There are three pairs of major glands: the parotid, submandibular and sublingual glands, and numerous minor salivary glands [37]. Salivary secretion continues throughout the day, with an average total volume of 500–600 mL. Previous studies have reported that the mean flow rate of unstimulated human saliva and stimulated human saliva is 0.35 mL/min and 2 mL/min, respectively [16][38][16,38].4. Drug Candidate and pH Modifier for Buccal/Sublingual Dosage Forms
4.1. Drug Candidate
The low drug loading capacity of buccal/sublingual formulations and the limited absorption area in the oral cavity are two main limitations for buccal/sublingual drug delivery. Thus, drug candidates should be high potency to achieve successful therapeutic efficacy. In addition, suitable drug candidates must not cause local irritation and toxicity at oral mucosa. Regarding physicochemical properties, high lipophilicity (log P (octanol/water) > 2), fairly good water-solubility and small molecular size (less than 800 Da) are typically considered as ideal parameters for drug candidates, as described previously [3]. The extent of different drug transport pathways across the epithelium depends on the drug physicochemical properties [39][40][43,44]. Typically, drug candidates with high lipophilicity can move across the lipid-rich epithelial cell membrane with relative ease. Fairly good water solubility allows for the fast drug release of buccal/sublingual formulations and drug diffusion across the hydrophilic cytoplasm of cells and paracellular passage. Macromolecules can be delivered via the oral mucosa, e.g., buccal insulin spray (Generex Oral-lyn®) was approved by Food and Drug Administration (FDA) for the treatment of patients under the Investigational New Drug (IND) program [41][42][43][45,46,47]. However, the number of marketed buccal/sublingual macromolecules is very small. However, over 40% of marketed drugs and approximately 90% of drug candidates are reported to be poorly water-soluble [5], and most of them are weakly ionizable drugs, indicating that their solubility and/or permeability across the lipid-rich epithelium are pH-dependent [44][45][46][47][49,50,51,52]. Typically, the ionic form of a drug is more water soluble than its non-ionic form. A change in the pH might influence the ratio of the ionized form of the dissolved drug, according to the Henderson-Hasselbach equation (Equation (4)) [48][53]. When the difference in the water solubility (and/or lipophilicity) between the two forms is big enough, a slight pH change might have a significant effect on the drug solubility. Therefore, drug candidates suitable for pHM modification should have pH-dependent solubility and/or pH-dependent lipophilicity and be poorly soluble at physiological pH in the oral cavity.4.2. pH Modifier
There are a few concerns about the excipients used in pharmaceutical formulations. A pH modifier can only be considered as a pharmaceutical excipient if it has been demonstrated to be safe for human beings. So far, various pH modifiers have been applied in the food and pharmaceutical industries. The Generally Recognized as Safe (GRAS) list of the FDA lists some safe pH modifiers that have been added to food. In addition, various pH modifiers recommended for oral liquids have been collected in the United States Pharmacopeia (USP). However, the specific pH modifiers for buccal/sublingual formulations were not referenced. The pH modifiers collected in the USP [49][67] and their maximum potency per unit dose used in solid oral and buccal/sublingual formulations in the database of Inactive Ingredient Search for Approved Drug Products Search, provided by the FDA [50][68]. The pH modifiers can be divided into three categories: acidifying agents, alkalizing agents and buffering agents. Currently, only a few pH modifiers, were applied in the commercial buccal/sublingual formulations approved by the FDA. pH modifiers demonstrated without local irritation and toxicity to oral mucosa could also be potential choices for the buccal/sublingual dosage forms.5. Methods for Microenvironmental pH Measurement
5.1. pH Electrode Approach
The most common method to determine the pHM is the pH electrode approach. As the previous studies described [51][52][53][54][55][56][57][57,77,78,79,80,81,82], formulations (e.g., tablets, film and patch) were allowed to swell in a limited volume of buffer solution (at neutral pH) at room temperature for a certain period. Subsequently, the pH on the surface of the formulations was determined using a pH electrode. Mucoadhesive buccal films containing ornidazole were allowed to swell in 4 mL of phosphate buffer (pH 6.8 ± 0.1) at room temperature for 120 min and the surface pH was measured using an electrode pH meter [52][77].5.2. Computer-Enhanced Color Images Method
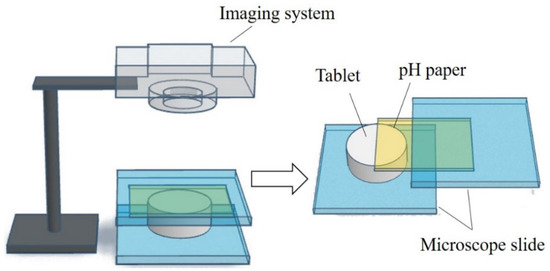
To gain more information on the pHM change during the dissolving process of the fentanyl tablet, a computer-enhanced color images (of pH paper) method was used to record the pHM as it varied over the surface of the swelling tablet [58][54]. The schematic view of the setup and the computer-enhanced color images of pH paper are shown in Figure 3. A piece of pH paper was placed over a tablet. The tablet with the pH paper was held between two microscope slides, and a small volume of deionized water was applied to the pH paper. The tablet was rapidly wetted by the water that permeated the pH paper. As the tablet swelled, the pH paper was digitally photographed at different time intervals. The pH over the distinct regions of the tablet surface were then determined from the digital images and in comparison to the reference pH standards. The pHM decreased from 7.0 to 5.0, and then gradually increased to around 6.0 during the first 5 min of the dissolving process [58][54].