Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Aliosha I. Figueroa-Valdés and Version 2 by Catherine Yang.
Small extracellular vesicles (sEVs) have burst into biomedicine as a natural therapeutic alternative for different diseases. Considered nanocarriers of biological origin, various studies have demonstrated the feasibility of their systemic administration, even with repeated doses. sEVs can resist the degradative conditions of the gastrointestinal tract after oral administration, accumulating regionally in the intestine, where they are absorbed for systemic biodistribution.
- small extracellular vesicles
- milk-derived vesicles
- food-derived vesicles
- exosomes
- oral delivery
- extracellular vesicles
1. Introduction
The enteral route, including oral administration of drugs is the preferred delivery method to treat systemic diseases or local gastrointestinal (GI) pathologies due to its minimal invasiveness (pain-free), relatively low cost, and ability to self-administer [1]. However, these advantages are challenged by acidic conditions in the stomach and degrading conditions in the intestine, which affect the stability, absorption, and bioavailability of various therapeutic molecules, limiting the diversity of therapeutic compounds that can be prescribed orally [2]. Indeed, macromolecules such as proteins, peptides, or nucleic acids as free agents show only slight absorption when administered orally, as they are degraded by GI enzymes, have low stability at acidic pH and limited permeation through biological barriers [3]. Likewise, several hydrophilic and lipophilic drugs also have limitations for their oral intake since their absorption is greatly conditioned by their molecular weight, logP value, and gastrointestinal permeability, often requiring nanocarriers to generate a biological effect [4].
Nanocarriers are a colloidal transport system for drugs with a nanometric particle size (typically < 500 nm) [5]. In their oral administration, they allow the safe transport of active therapeutic molecules, improving pharmacokinetics, biodistribution, and stability, reducing toxicities, and offering a controlled release and delivery of drugs to specific sites [5]. Small extracellular vesicles (sEVs) are one of the most studied nanocarriers due to their biological origin that endows them with different and natural attributes that favor their biomedical use [6]. Defined as cell-derived nanostructures enclosed in a lipid membrane, they transport and protect an active molecular cargo composed of nucleic acids, proteins, and lipids [7][8][9][10][7,8,9,10]. In the physiologic and pathologic processes, they play a role in the regulation of intercellular communication [11][12][11,12]. As nanocarriers, they have advantageous attributes related to their proven rapid internalization, low immunogenicity even at repeated doses, high stability in physiological conditions, and the capability of modifying their internal and superficial components to generate a controlled and specific release of endogenous or loaded therapeutic molecules [13]. Compared with synthetic nanocarriers, sEVs exhibit substantial benefits in targeting, safety, and pharmacokinetics, being considered the next-generation drug delivery platform [14].
Since its introduction as a drug delivery nanosystem, research has focused on understanding the pharmacokinetics and biodistribution of intravenously (i.v.) administered sEVs [15]. The valuable information collected on this subject contrasts with the need for more knowledge about the safety, stability, pharmacokinetics, and biodistribution of orally administered sEVs, the preferred route of administration for doctors and patients. Although there are few quality studies, the data to date show that, remarkably, sEVs can withstand the harsh environment of the GI tract and reach the intestine, where they accumulate heavily. It is from the intestinal lumen where sEVs penetrate the epithelium, which is the innermost layer lining the entire GI tract and selectively regulates transport from the lumen to the underlying tissue compartment [1]. Although most of these studies are limited to a single source of sEVs, cow’s milk, they remarkably demonstrate the efficacy of using sEVs as a nanocarrier system for a therapeutic payload to elicit a desired biological effect.
2. Challenges of Orally Administered sEVs
sEVs are non-replicative lipid-based vesicles classified by a hydrodynamic diameter inferior to 200 nanometers (nm) [16]. They are secreted by most known cells and can be found in every biological fluid (blood, urine, saliva, breast milk, among others) [13]. Besides, their biogenesis mechanism allows the transport and protection of bioactive molecules (nucleic acids, proteins, lipids, or metabolites) from a donor towards an acceptor cell, modifying their transcriptional profile, function, or phenotype [13]. Unique properties such as high relative stability, biocompatibility, permeability, low toxicity, and low immunogenicity determine its success as a novel cell-free therapeutic agent. Their versatile properties may be modified by different bioengineering allowing the insertion of targeting motifs for specific cellular lineages in their surface, a load of therapeutic drugs or macromolecules in their membrane or lumen, or even they can be modified to increase blood circulation time (Figure 1) [17].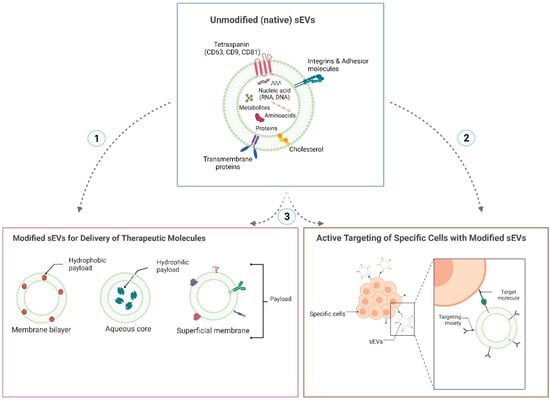
Figure 1. Diagram of the native structure of small extracellular vesicles and the functionalization strategies that can be performed on them to provide them with specific therapeutic properties. Small extracellular vesicles (sEVs) have a structure formed by a membrane composed of a lipid bilayer. Different proteins are expressed in it, which may be common to the vast majority of sEVs (such as tetraspanins for sEVs derived from eukaryotic cells) or specific proteins according to the origin of their parental cell. The core of sEVs is composed of nucleic acids, lipids, proteins, and metabolites. One of the characteristics of sEVs that make them good nanocarriers is that they can be easily modified to endow them with specific therapeutic properties. For example, to acquire a certain therapeutic efficacy, sEVs can be engineered to carry a specific therapeutic payload: drugs, proteins, or different types of nucleic acids (siRNA, miRNA, shRNA). Depending on the molecule’s type and therapeutic function to be triggered, the payload can be incorporated into or anchored to the surface of the sEVs membrane. It can also be loaded into the sEVs core (1). To provide them with a better safety profile, sEVs can be functionalized to target a specific cell or tissue by incorporating a targeting moiety into their surface membrane (2). This strategy reduces off-target interactions while improving the bioavailability of the therapeutic molecule at the site of interest. Both the strategy of therapeutic loading molecules and the strategy of targeting sEVs to a specific tissue can be performed together in sEVs (3), providing the nanovesicles with better efficacy and safety profiles at the same time (created with http://www.biorender.com (accessed on 16 november 2022)).
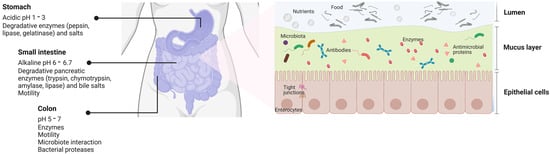
Figure 2. Scheme of the gastrointestinal tract and the physiological factors that influence the absorption of therapeutic molecules. Several physiological barriers in the gastrointestinal (GI) tract challenge drug administration by the oral route. In the GI environment, the presence of factors such as pH, degradative enzymes and salts, motility and interaction with the microbiota can alter the solubility and stability of drugs, which finally affect their permeability across the mucosal barriers. This figure is based on a schematic drawing and does not fully represent the accurate structural reality of the intestine (created with http://www.biorender.com (accessed on 21 november 2022)).
3. Biodistribution, Stability, and Safety of Oral Delivery of Native and Drug Loaded sEVs
Murine studies identifying the biodistribution pattern of orally administered sEVs are few and focus mainly on cow’s milk-derived sEVs, although some studies using plant-derived exosomes-like particles. These studies show that sEVs/exosomes-like particles manage to withstand the hostile environment of the gastrointestinal tract, associated with their transit through acidic conditions in the stomach and degradative conditions in the gut, in various murine models [24][25][26][24,25,26]. Cow’s milk-derived sEVs cross the upper gastrointestinal tract and reach the intestine in relatively short times (1–6 h) [27][28][27,28]. The absorption of sEVs seems to occur in the gut through mechanisms that are not well understood, but that facilitates the entry of sEVs into the systemic circulation and their distribution in other organs, essentially localized in the abdominal cavity [27][28][29][30][31][27,28,29,30,31]. Unlike the “trapping” of sEVs in the organs of the mononuclear phagocytic system (liver, spleen, and lung) after systemic injection of sEVs [17], oral ingestion allows a considerable accumulation of sEVs in the intestine [27][28][30][32][33][34][27,28,30,32,33,34]. In the other organs of the body, the accumulation of sEVs is less but notably shows a homogeneous distribution among them. Figure 3 shows the biodistribution pattern in mice after oral and systemic administration.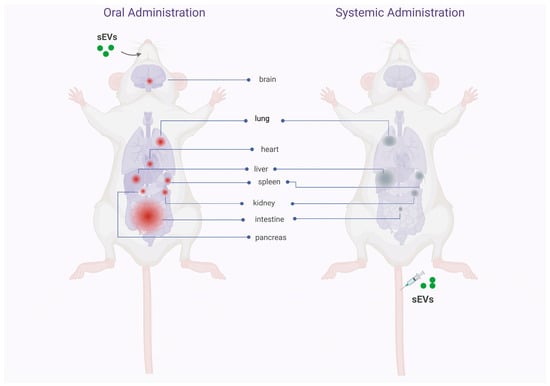
Figure 3. Comparative diagram of the biodistribution pattern of sEV administered orally and intravenously. The illustration shows the pattern of biodistribution of sEVs in different mice tissues after oral or intravenous administration. In the body on the left, the tissues and organs where the sEV would accumulate after intestinal absorption are identified in red. The considerable accumulation of sEVs in the intestine and, to a lesser extent, in the rest of the body’s organs stands out. In the body on the right, the organs where sEVs would accumulate after intravenous administration are identified in gray. A considerable accumulation of sEVs is observed in the organs associated with the mononuclear phagocytic system (liver, spleen, lung), with little reach to other body organs. These data suggest that the biodistribution pattern is defined by the route of administration of the sEVs, a dependency that can be used strategically to reach a specific organ in patients (created with http://www.biorender.com (accessed on 2 february 2023)).
4. Food Derived Vesicles (FDVs)-Based Nutraceutical Perspectives in Infant and Elderly Health
In the last decade, structures morphologically like extracellular vesicles (EVs), called “food-derived vesicles” or FDVs, have been isolated from different foods (such as honey, pollen, milk, fruits, and vegetables, among other foods). These findings raise the question of whether FDVs contain or overexpress the nutritional compounds or nutraceutical effects of the foods from which they are derived. Several studies have shown that different FDVs have nutraceutical effects. For example, it was observed that the nanovesicles in Apis mellifera hypopharyngeal gland secretomal products (honey, royal jelly, and bee pollen) participate in the known antibacterial and pro-regenerative properties of bee-derived products [35][62]. Furthermore, Chen et al. [36][63] described that honey-derived nanoparticles possess anti-inflammatory properties by inhibiting the NLRP3 inflammasome, thus preventing liver damage in vivo. As the research mentioned, various other studies report the presence of FDVs and their bioactive compounds in different models of diseases such as cancer, intestinal inflammation, and autoimmune diseases [37][64]. In global terms, the biological effect reported for FDVs is highly associated with the source of identification. However, comparing articles is challenging since different isolation methods are employed, the doses administrated are different, and several routes of administration are tested [37][38][36,64].
Several studies have revealed the effects of sEVs derived from breast milk on the immune function of infants. The analysis of breast milk-derived sEVs showed that the molecules they contain vary depending upon the maternal allergy status [39][65]. Malnutrition in the elderly population is an important risk factor for sarcopenia, osteoporosis, and other age-related diseases. Protein and other components are key nutrients for the human body and affect bone and muscle mass and quality. Dairy products are rich in these nutrients, which implies that dairy products or their bioactive components, such as sEVs might be ideal for the elderly population. The use of milk sEVs as bioactive ingredients represents a novel avenue to explore in the context of human nutrition, and they might exert significant beneficial effects at multiple levels, including but not limited to intestinal health, bone and muscle metabolism, immunity, modulation of the microbiota, growth and development [40][66].
