Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by László Baranyai.
In the postharvest preservation stage, fruits undergo various technical treatments for maintaining their quality. A recently adopted technology is the application of edible coatings, which can be applied to a diverse range of fruits to regulate the exchange of moisture and gases between the fruit and its environment.
- fresh fruit
- postharvest technology
- fruit packaging
- fruit quality
- preservation
1. Characterizations of Edible Coatings
Initially, edible coatings were developed to replace and decrease the usage of other kinds of chemicals and synthetic compounds that may be harmful to customers’ health. An edible coating is a covering sheet composed of biological or chemical ingredients and utilized as a monolayer film or multilayer films on the surface of the product [29][1]. Edible coatings help maintaining the phytonutrients (antioxidants, phenolics, pigments) and controlling physicochemical qualities (inhalation and exhalation rate, weight loss, total dissolved matters, pH) of fruits for longer time [30][2]. As a result, fruit deterioration is delayed, fruit quality is improved, and fruit shelf life is extended [31][3]. To be effective, edible coatings must meet several functional requirements (Figure 1), including (i) being free of toxic materials and harmless for human beings; (ii) having superb boundary capabilities regarding water, humidity, O2, CO2, and C2H4; and (iii) improving the visual as well as textural properties of the coated products [32][4]. The coating should not alter the sensory properties of the fruit [33][5]. Therefore, edible coating formula needs to be carefully considered during development. Furthermore, the coating should control the gas exchange to avoid fruit fermentation and undesirable off-flavor [12][6]. In the case of fruit coating, when the oxygen level falls below 3%, anaerobic respiration takes over, which generates undesirable flavors and induces other issues such as colorant and structure alterations. Therefore, high concentration of O2 (>8%) and low concentration of CO2 (<5%) are recommended to prevent or delay deterioration, hence preserving food quality [34][7].
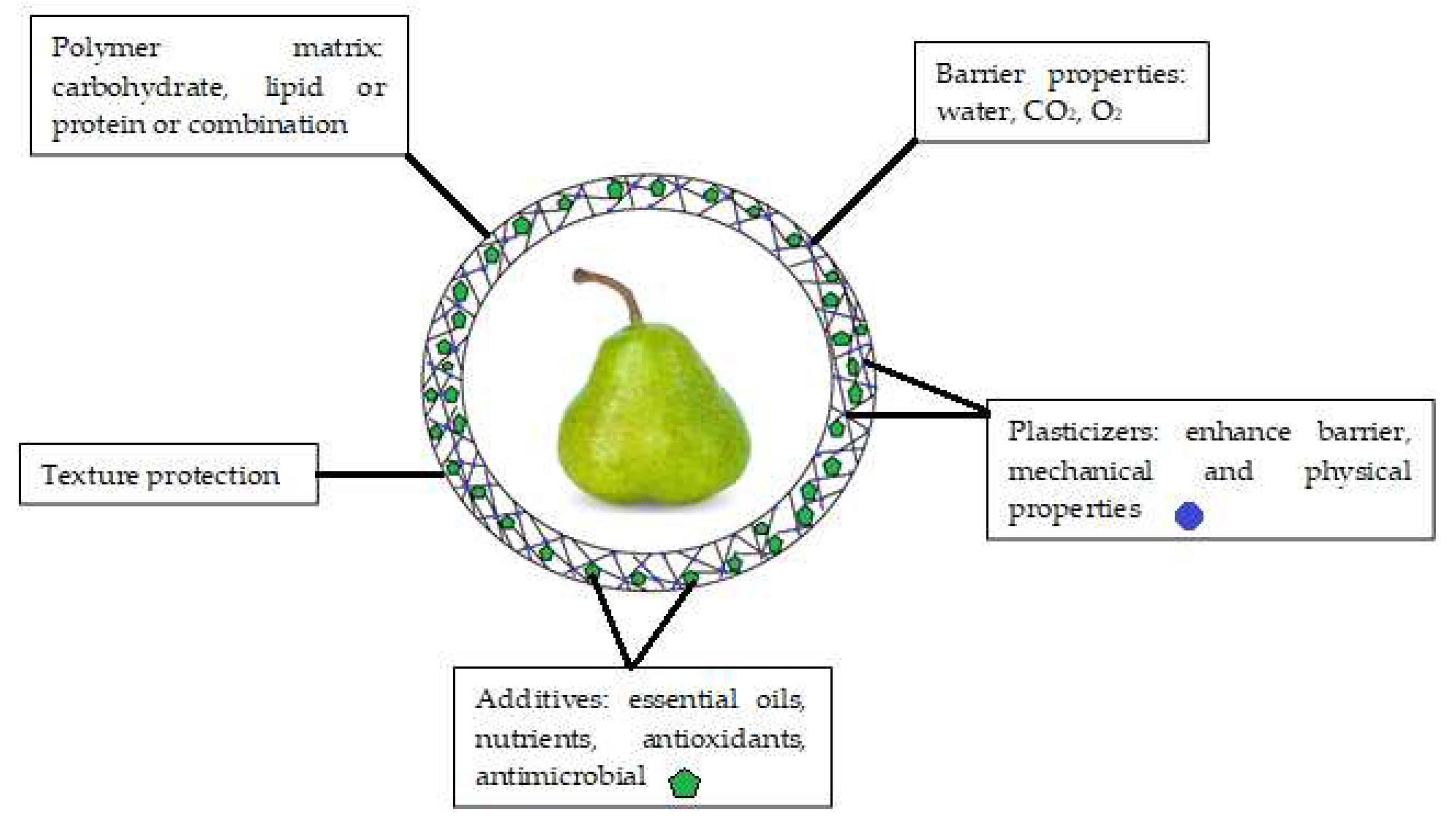
Figure 1.
Main components and functions of edible coatings.
The coating-forming solutions can comprise a single main component from proteins, polysaccharides, lipids, or a mixture of them to achieve desired properties [35][8]. New edible coating candidates should be inexpensive and available in large quantity. The coating should provide easy application, have good adhesive characteristics, and dry quickly with uniform thickness. Moreover, the coating performance and structural stability must be maintained during long-term storage. The coating must be flexible enough to adapt to specific morphological changes such as fruit shrinkage or mechanical damage [36][9].
The characteristics of coating polymers determine their use and function [37][10]. Proteins and polysaccharides establish strong molecular interactions in polymers. They have superb mechanical and gas isolation (oxygen and carbon dioxide) qualities that inhibit the common ripening process in many fruits [38,39][11][12]. The most universally used polysaccharides in food production can be attributed to cellulose and its derivatives, namely pectin, chitosan, and gums [40][13]. However, studies showed that the drawback of their application is the poor performance in preventing fruit moisture loss [41][14]. In addition, usage of proteins in edible coatings may be limited because they are potentially allergenic or refused due to religious belief [42][15]. Coatings made from lipids (fatty acids, acylglycerol or waxes) are great moisture barriers due to their hydrophobic property [36][9]. Unfortunately, coatings based on lipids were found to be poor in mechanical attributes and brittleness due to their lack of cohesiveness and structural integrity [43][16]. Lipid molecules are frequently added to matrices to reduce the water sensitivity of a hydrocolloid-based coating [43][16]. Therefore, mixtures of different components can be used for production of edible coatings that enhance the physicochemical characteristics and solve the drawbacks of the individual components [44][17].
Coatings that are based on polysaccharides and proteins achieved excellent barrier characteristics. However, they are also less flexible due to strong intermolecular forces along the polymer chain. As a result, blisters, flakes, or cracks may appear in the coating as the fruit shrinks during storage [33][5]. The primary role of plasticizers is to strengthen coating flexibility and decrease brittleness. Glycerol, mannitol and sorbitol are the food grade plasticizers typically used in edible coatings [45][18]. In addition, sugars with small molecules (such as fructose-glucose syrups and honey), other polyols (such as glycerol derivatives and propylene glycols), lipids and by-products (such as phospholipids, fatty acids, lecithin, oils, and waxes), and water are also popular examples of food grade plasticizers that can be added to coatings [46][19]. The plasticizer absorbs more water into the coating matrix and decreases the intermolecular interactions along the polymer chain, thereby preventing the coating from blistering, flaking and cracking [47,48][20][21]. Furthermore, the amount of plasticizer used in the coating formulation must be examined according to its influence on the permeability of the wrapping. Plasticizers increase the moisture and gas permeability through the coating by reducing polymer interactions and increasing intermolecular space [47][20]. Even though plasticizers are supposed to give the coatings elastic structure, overusing plasticizers results in decreased moisture resistance and weaker mechanical strength. Table 1 summarizes the advantages and challenges of plasticizers reported in edible coatings of fruits.
Table 1.
Effect of plasticizers on the characteristics of edible coatings.
| Fruits | Coating | Advantages | Challenges | References |
|---|---|---|---|---|
| Plum | Hydroxypropyl methylcellulose−beeswax with glycerol (G) or mannitol (M) | The water vapor permeability (WVP) and flexibility increased together with G content, whereas the brittleness was affected by increased M content but not WVP. | The flexibility and WVP of G-plasticized coatings decreased as beeswax content rose. | [47][20] |
| Fresh-cut apples | Apple-puree-based edible coatings with glycerol | The coating became more flexible and had less adhesion to the casting surface after being mixed with G. | The WVP of coatings increased together with G content. | [49][22] |
| Guava | Cassava starch, casein, and gelatin with sorbitol (S) | The flexibility of coatings has been enhanced by adding S. | The solubility was increased. | [50][23] |
| Tomato | Mango kernel starch with glycerol and sorbitol | Sorbitol-containing coating formulation was shown to be the most efficient, succeeded by mixed plasticizers (glycerol/sorbitol) and finally glycerol alone. | Increased glycerol led to increased WVP and oxygen permeability. | [51][24] |
When using hydrophilic polyols (such as glycerol or sorbitol) as plasticizers, the water solubility, elongation, and the moisture permeability of coatings were substantially improved following the plasticizer concentration. The observed total color difference and the puncture strength, on the other hand, decreased with higher plasticizer content. The mechanical properties of puncture strength and elongation of coatings are more affected by glycerol than sorbitol. In addition, sorbitol-plasticized coatings had decreased water vapor permeability, which can be increased with higher plasticizer concentration. However, this rise was less than that found with glycerol-plasticized coatings [52][25]. Additionally, Yang and Paulson [53][26] found that sorbitol did not show a plasticizing effect on gellan films, although it had been widely used in protein-based films. That study showed that polyethylene glycol and glycerol were effective in plasticizing gellan films at 60% concentration. It has been proved that at lower concentration, the films turned to be more fragile, and their manipulation became challenging, while glycerol concentration beyond 75% results in sticky behavior. Additionally, the presence of plasticizers promoted the homogenous coating structure, preventing phase separation. By limiting the development of pores or cracks, the integrity of the coating can be maintained [54][27]. The coatings with low protein content in the formulation showed smoother surfaces when treated with plasticizers [55][28].
2. Application Technology of Edible Coating
Following the selection of the wrapping composition, the application of the solution to the surface of the fruit is the next important step. There are different approaches about how to cover the food surface with edible coating (Figure 2). Dipping is the simplest method consisting of three steps: (i) immersion and dwelling, (ii) deposition and (iii) evaporation of solvents [56][29]. After the excess solution has been drained away, the food is typically dried at ambient condition or treated with a dryer [11][30]. Previous studies showed that the density and morphology of coatings precipitated by dipping are significantly affected by several factors including time for immersion, speed of withdrawal, number of cycles for dip-coating, coating solution parameters such as density, viscosity, surface tension, substrate surface characteristics, and drying conditions [57][31]. However, there are numerous disadvantages of the dipping method. Dipping commonly results in a layer with heavy thickness leading to substantially reduced fruit respiration, damage food surfaces and degraded function. In addition, microorganisms and dirt from the fruit surface may contaminate the coating solution, hence challenging the industrial up-scaling. Another drawback of the dipping approach is the large quantity of solution needed for coating per unit mass of product to guarantee optimum dipping conditions [33][5].
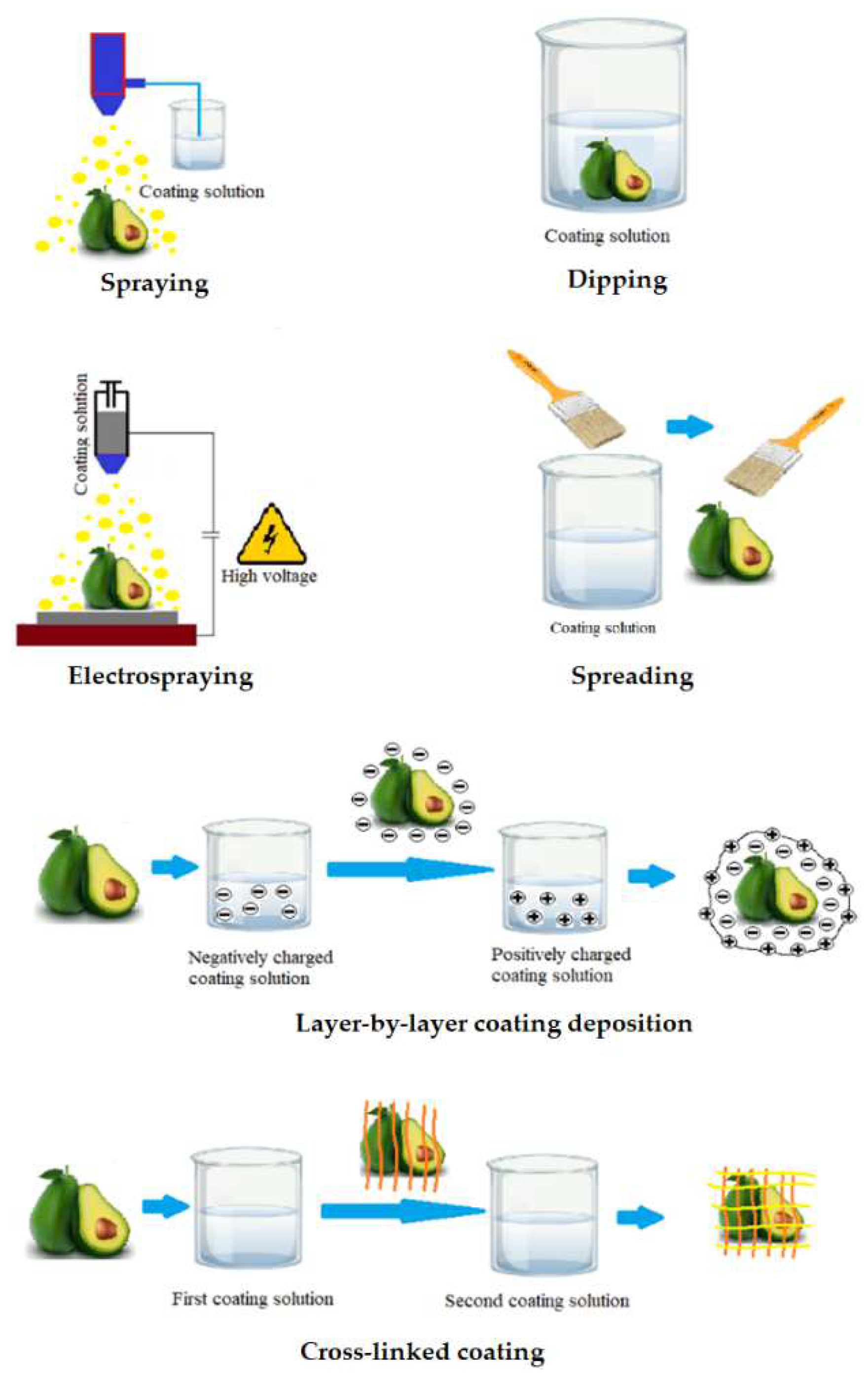
Figure 2.
Application methods of edible coating.
The spreading technique is effective for coating solutions with high viscosity. In general, the wetting level and spreading rate are the key factors used to describe how the coating solution is spread across the food surface. Several parameters influence the efficiency of coating deposition by spreading, including substrate quality, particularly drying conditions, liquid characteristics, and surface geometry [58][32]. Specialized operators and technicians typically perform brushing. Thus, the human factor has a significant impact on the quality of coating and thickness homogeneity.
Spraying is the process of using a set of nozzles to distribute small droplets on the fruit surface. Spraying methods are used in three different ways, including air spray atomization, pressure atomization and air assisted airless atomization [11][30]. The spraying technology allows multi-layer applications such as interlayer solutions, as well as consistent coating with homogeneous thickness [59][33]. Additionally, the coating thickness is greater than that of dipping method due to the low viscosity of the solution [11][30].
The electrospraying method uses a strong electric field to produce charged droplets, with a very narrow size distribution, that are micrometric and sub-micrometric in size [60][34]. The electrospraying process can adjust droplet specifications such as size, or produced layer thickness by controlling the flow rate as well as the viscosity of the solution [61][35].
The layer-by-layer deposition method is based on the electrostatic interactions of the food surface with charged polyelectrolytes. These electrostatic interactions improve adhesion of the coating to the food surface and may be used to create coatings with two or more thin layers that are chemically or physically linked to each other. Such linked multilayer coating improves the effectiveness compared to conventional edible coatings [62][36]. The use of the multilayer coating approach to increase the compactness of the coating layers during postharvest storage of fruits were documented for polysaccharides and charged polyelectrolytes capable of hydrogen and covalent bonding. Polysaccharides and charged polyelectrolyte demonstrated efficiency in fruit preservation when applying the multi-layer coating method to improve the tightness of the coatings [63][37].
The final technique in the list is cross-linking, which is defined as the process of combining polymer chains using covalent and non-covalent linkages. Cross-linked coatings are typically created by spraying, dipping, or spreading the coating solution onto the food surface. A cross-linking agent is then added to provide a more compact and stable coating. Cross-linked coatings have substantial benefits, including better mechanical properties, chemical and thermal stability as well as better molecular migration [64][38]. Cross-linking is particularly effective for biopolymer materials formed from proteins or polysaccharides. Proteins are used more frequently than polysaccharides with this technique due to the greater number of functional groups in proteins [37][10].
The results of different application techniques for edible coatings are listed in Table 2. The findings in the list confirm the idea that coating technology in postharvest preservation of fruits can be considered a sustainable solution.
Table 2.
Edible coatings applied to fruits.
| Method of Application |
Coated Fruits | Coating Matrix | Results | References |
|---|---|---|---|---|
| Dipping | Blueberries | Chitosan Blueberry fruit and leaf extracts (antioxidants, antimicrobials) |
Greatly increased shelf life; decreased microbial growth and degradation rate. | [65][39] |
| Oranges | Gelatin Persian gum and shellac (antioxidants) |
Reduced weight loss, reduced titratable acidity (TA), increased total phenolic contents (TPC) and antioxidant capacity (AOC), and preserved fruit firmness and gloss. | [66][40] | |
| Pineapples | Sodium alginate Citral nano-emulsions: anti-browning agents, antioxidants, antimicrobials |
Reduced microbiological growth, lower respiration rate, and improved color retention. | [67][41] | |
| Strawberry | Candelilla wax with Bacillus subtilis | Maintained total soluble solid content and pH, inhibited R. stolonifer | [68][42] | |
| Guava | Chitosan blended with alginate NanoZnO |
Reduced the change in weight loss and color. Shelf life was increased more than 13 days | [69][43] | |
| Avocado | Sodium alginate combined with Meyerozyma caribbica | Decreased weight loss rate and inhibited C. gloeosporioides | [70][44] | |
| Spreading | Fresh-cut apples | Sunflower with olive oil Ascorbic acid with lecithin |
Improved color retention. | [71][45] |
| Figs | Chitosan Acetic acid, cinnamon essential oil with canola oil and Rosselle extract |
Reduced fungus growth, delayed color change and more retained antioxidant capacity. | [72][46] | |
| Papaya | Carnauba wax nanoemulsion and Hydroxy methyl propyl cellulose |
Weight loss was decreased while color was retained | [73][47] | |
| Spraying | Raspberries | Gelatin Propolis extracted in ethanol and zein nanocapsules (antimicrobials) |
Increased shelf life and antifungal activity. | [74][48] |
| Kiwi | Hydroxypropyl methyl cellulose Essential oils of lemon and aloe vera gel |
Enhanced firmness, brightness, greenness, and total soluble solids (TSS) while minimized weight loss, and browning, decreased microbial load. | [15][49] | |
| Orange | Carnauba wax combined with orange peel essential oil and montmorillonite nanoclay | Extened shelf life and maintained vitamin C content | [75][50] | |
| Electrospraying | Fresh-cut apples | W/O emulsions: Refined olive oil with polyglycerol polyricinoleate | Lower weight loss and better color maintenance compared to the dip-coated samples. | [76][51] |
| Strawberries | W/O emulsions: Refined olive oil with polyglycerol polyricinoleate | Reduced moisture loss, improved firmness and color maintenance. | [77][52] | |
| Banana | Ethylene scavenger films combined with zein-Artemisia sphaerocephala Krasch | Reduced the rate of browning and loss of hardness. | [78][53] | |
| Layer-by-layer | Fresh-cut apples | Carboxymethylcellulose sodium salt (NaCMC) combined with chitosan | Good inhibition of browning, weight loss, and metabolic activity. | [79][54] |
| Citrus fruit | Carboxymethyl cellulose (CMC) combined with chitosan | Greatly enhanced fruit glossiness and appearance, not very effective in preventing weight loss. | [80][55] | |
| Mango fruits | Polystyrene sulfonate sodium salt (PSS) combined with poly diallyl dimethylammonium chloride (PDADMAC) | Improved hydrophilicity of the outer surface. | [81][56] | |
| Pear | Chitosan combined with alginate | Extended shelf life, maintained firmness and color | [82][57] | |
| Cross-linked coating | Rose apple | Sodium alginate solution with CaCl2 solution | Greatly decreased weight loss and respiration rate, improved appearance | [83][58] |
| Fresh-cut mango | Protein/guar gum and mango puree/calcium chloride | The quality was maintained for 15 days. The crosslinked protein coating was more effective than native protein coating | [30][2] | |
| Sweet cherries | Alginate/Oil nanoemulsion | Improved qualities, reduced cracking on fruits | [84][59] |
References
- Paidari, S.; Zamindar, N.; Tahergorabi, R.; Kargar, M.; Ezzati, S.; Musavi, S.H. Edible coating and films as promising packaging: A mini review. J. Food Meas. Charact. 2021, 15, 4205–4214.
- Sharma, P.; Shehin, V.P.; Kaur, N.; Vyas, P. Application of edible coatings on fresh and minimally processed vegetables: A review. Int. J. Veg. Sci. 2019, 25, 295–314.
- Irimia, A.; Stoleru, E.; Vasile, C.; Bele, A.; Brebu, M. Application of vegetal oils in developing bioactive paper-based materials for food packaging. Coatings 2021, 11, 1211.
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.; McHugh, T.H. Recent advances on edible films based on fruits and vegetables—A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169.
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75.
- Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170.
- Kader, A.A. Biochemical and physiological basis for effects of controlled and modified atmospheres on fruits and vegetables. Food Technol. 1986, 40, 99–104.
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides; lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107.
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag. Shelf Life 2018, 16, 157–167.
- Dai, L.; Zhang, J.; Cheng, F. Cross-linked starch-based edible coating reinforced by starch nanocrystals and its preservation effect on graded Huangguan pears. Food Chem. 2020, 311, 125891.
- Azeredo, H.M.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact–A review. Trends Food Sci. Technol. 2016, 52, 109–122.
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302.
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res. Int. 2020, 137, 109376.
- Mederos-Torres, Y.; Bernabe-Galloway, P.; Ramirez-Arrebato, M.A. Polysaccharide-based films as biodegradable coatings in fruits postharvest. Cultiv. Trop. 2020, 41.
- Arnon-Rips, H.; Poverenov, E. Improving food products' quality and storability by using Layer by Layer edible coatings. Trends Food Sci. Technol. 2018, 75, 81–92.
- De Castro e Silva, P.; de Oliveira, A.C.; Pereira, L.A.; Valquíria, M.; Carvalho, G.R.; Miranda, K.W.; Oliveira, J.E. Development of bionanocomposites of pectin and nanoemulsions of carnauba wax and neem oil pectin/carnauba wax/neem oil composites. Polym. Compos. 2020, 41, 858–870.
- Shigematsu, E.; Dorta, C.; Rodrigues, F.J.; Cedran, M.F.; Giannoni, J.A.; Oshiiwa, M.; Mauro, M.A. Edible coating with probiotic as a quality factor for minimally processed carrots. J. Food Sci. Technol. 2018, 55, 3712–3720.
- Hershko, V.; Klein, E.; Nussinovitch, A. Relationships between edible coatings and garlic skin. J. Food Sci. 1996, 61, 769–777.
- Han, J.H. Edible films and coatings: A review. In Innovations and Food Packaging; Han, J.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 213–255. ISBN 978-0-12-394601-0.
- Navarro-Tarazaga, M.L.; Sothornvit, R.; Pérez-Gago, M.B. Effect of plasticizer type and amount on hydroxypropyl methylcellulose− beeswax edible film properties and postharvest quality of coated plums (cv. Angeleno). J. Agric. Food Chem. 2008, 56, 9502–9509.
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures; active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303.
- McHugh, T.H.; Senesi, E. Apple wraps: A novel method to improve the quality and extend the shelf life of fresh-cut apples. J. Food Sci. 2000, 65, 480–485.
- Pellá, M.C.; Silva, O.A.; Pellá, M.G.; Beneton, A.G.; Caetano, J.; Simões, M.R.; Dragunski, D.C. Effect of gelatin and casein additions on starch edible biodegradable films for fruit surface coating. Food Chem. 2020, 309, 125764.
- Nawab, A.; Alam, F.; Hasnain, A. Mango kernel starch as a novel edible coating for enhancing shelf-life of tomato (Solanum lycopersicum) fruit. Int. J. Biol. Macromol. 2017, 103, 581–586.
- Ballesteros-Mártinez, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Effect of glycerol and sorbitol concentrations on mechanical; optical; and barrier properties of sweet potato starch film. NFS J. 2020, 20, 1–9.
- Yang, L.; Paulson, A.T. Mechanical and water vapour barrier properties of edible gellan films. Food Res. Int. 2000, 33, 563–570.
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356.
- Al-Hassan, A.A.; Norziah, M.H. Starch–gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117.
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374.
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing spray systems for application of edible coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337.
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism; methods and applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404.
- Kumar, G.; Prabhu, K.N. Review of non-reactive and reactive wetting of liquids on surfaces. Adv. Colloid Interface Sci. 2007, 133, 61–89.
- Martín-Belloso, O.; Rojas-Graü, M.A.; Soliva-Fortuny, R. Edible Films and Coatings for Food Applications; Embuscado, M.E., Huber, K.C., Eds.; Springer: New York, NY, USA, 2009; pp. 295–313.
- Khan, M.K.I.; Schutyser, M.; Schroën, K.; Boom, R. Barrier properties and storage stability of edible coatings prepared with electrospraying. Innov. Food Sci. Emerg. Technol. 2014, 23, 182–187.
- Jaworek, A.T.S.A.; Sobczyk, A.T. Electrospraying route to nanotechnology: An overview. J. Electrost. 2008, 66, 197–219.
- Skurtys, O.; Acevedo, C.; Pedreschi, F.; Enrione, J.; Osorio, F.; Aguilera, J.M. Food hydrocolloid edible films and coatings. In Food Hydrocolloids: Characteristics; Properties and Structures; Hollingworth, C.S., Ed.; Nova Science: Hauppauge, NY, USA, 2010; pp. 41–80. ISBN 9781608762224.
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as edible films and coatings: Characteristics and influence on fruit and vegetable quality—A review. Agronomy 2021, 11, 813.
- Guo, Q.; Paliy, M.; Kobe, B.; Trebicky, T.; Suhan, N.; Arsenault, G.; Yang, J. Characterization of cross-linking depth for thin polymeric films using atomic force microscopy. J. Appl. Polym. Sci. 2015, 132, 41493.
- Yang, G.; Yue, J.; Gong, X.; Qian, B.; Wang, H.; Deng, Y.; Zhao, Y. Blueberry leaf extracts incorporated chitosan coatings for preserving postharvest quality of fresh blueberries. Postharvest Biol. Technol. 2014, 92, 46–53.
- Khorram, F.; Ramezanian, A.; Hosseini, S.M.H. Shellac; gelatin and Persian gum as alternative coating for orange fruit. Sci. Hortic. 2017, 225, 22–28.
- Prakash, A.; Baskaran, R.; Vadivel, V. Citral nanoemulsion incorporated edible coating to extend the shelf life of fresh cut pineapples. LWT 2020, 118, 108851.
- Oregel-Zamudio, E.; Angoa-Pérez, M.V.; Oyoque-Salcedo, G.; Aguilar-González, C.N.; Mena-Violante, H.G. Effect of candelilla wax edible coatings combined with biocontrol bacteria on strawberry quality during the shelf-life. Sci. Hortic. 2017, 214, 273–279.
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; de Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566.
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Sandoval-Contreras, T.; Calderón-Santoyo, M. Sodium alginate coatings added with Meyerozyma caribbica: Postharvest biocontrol of Colletotrichum gloeosporioides in avocado (Persea americana Mill. cv. Hass). Postharvest Biol. Technol. 2020, 163, 111123.
- Khan, M.K.I.; Cakmak, H.; Tavman, Ş.; Schutyser, M.; Schroёn, K. Anti-browning and barrier properties of edible coatings prepared with electrospraying. Innov. Food Sci. Emerg. Technol. 2014, 25, 9–13.
- Saavedra, S.C.; Ventura-Aguilar, R.I.; Bautista-Baños, S.; Barrera-Necha, L.L. Biodegradable chitosan coating for improving quality and controlling Alternaria alternata growth in figs. World J. Adv. Res. Rev. 2020, 7, 115–125.
- Miranda, M.; Gozalbo, A.M.; Sun, X.; Plotto, A.; Bai, J.; de Assis, O.B.G.; Ferreira, M.D.; Baldwin, E. Effect of mono and bilayer of carnauba wax based nano-emulsion and HPMC coatings on post-harvest quality of 'redtainung' papaya. In Proceedings of the SIAGRO 2019 Simpósio Nacional de Instrumentação Agropecuária, São Carlos, Brazil, 3–5 December 2019; p. 5.
- Moreno, M.A.; Vallejo, A.M.; Ballester, A.R.; Zampini, C.; Isla, M.I.; López-Rubio, A.; Fabra, M.J. Antifungal edible coatings containing Argentinian propolis extract and their application in raspberries. Food Hydrocoll. 2020, 107, 105973.
- Passafiume, R.; Gaglio, R.; Sortino, G.; Farina, V. Effect of three different aloe vera gel-based edible coatings on the quality of fresh-cut “hayward” kiwifruits. Foods 2020, 9, 939.
- Nasirifar, S.Z.; Maghsoudlou, Y.; Oliyaei, N. Effect of active lipid-based coating incorporated with nanoclay and orange peel essential oil on physicochemical properties of Citrus sinensis. Food Sci. Nutr. 2018, 6, 1508–1518.
- Cakmak, H.; Kumcuoglu, S.; Tavman, S. Production of edible coatings with twin-nozzle electrospraying equipment and the effects on shelf-life stability of fresh-cut apple slices. J. Food Process Eng. 2018, 41, e12627.
- Cakmak, H.; Kumcuoglu, S.; Tavman, S. Electrospray coating of minimally processed strawberries and evaluation of the shelf-life quality properties. J. Food Process Eng. 2019, 42, e13082.
- Fan, X.; Rong, L.; Li, Y.; Cao, Y.; Kong, L.; Zhu, Z.; Huang, J. Fabrication of bio-based hierarchically structured ethylene scavenger films via electrospraying for fruit preservation. Food Hydrocoll. 2022, 133, 107837.
- Liu, X.; Han, W.; Zhu, Y.; Xuan, H.; Ren, J.; Zhang, J.; Ge, L. Anti-oxidative and antibacterial self-healing edible polyelectrolyte multilayer film in fresh-cut fruits. J. Nanosci. Nanotechnol. 2018, 18, 2592–2600.
- Arnon, H.; Zaitsev, Y.; Porat, R.; Poverenov, E. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol. Technol. 2014, 87, 21–26.
- Kittitheeranun, P.; Dubas, S.T.; Dubas, L. Layer-by-layer surface modification of fruits with edible nano-coatings. Appl. Mech. Mater. 2012, 229, 2745–2748.
- Hira, N.; Mitalo, O.W.; Okada, R.; Sangawa, M.; Masuda, K.; Fujita, N.; Kubo, Y. The effect of layer-by-layer edible coating on the shelf life and transcriptome of ‘Kosui’Japanese pear fruit. Postharvest Biol. Technol. 2022, 185, 111787.
- Duong, N.T.C.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Kaisangsri, N. An innovative single step of cross-linked alginate-based edible coating for maintaining postharvest quality and reducing chilling injury in rose apple cv.'Tabtimchan'(Syzygium samarangenese). Sci. Hortic. 2022, 292, 110648.
- Gutiérrez-Jara, C.; Bilbao-Sainz, C.; McHugh, T.; Chiou, B.S.; Williams, T.; Villalobos-Carvajal, R. Effect of cross-linked alginate/oil nanoemulsion coating on cracking and quality parameters of sweet cherries. Foods 2021, 10, 449.
More
