You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Yang Wang.
MOFs are a class of porous materials with a three-dimensional frame structure shaped by the self-assembly of metal centers and organic ligands. Because of its unique performance advantages, it has become a promising adsorbent.
- MOFs
- functional groups
- pollutants
- water purification
1. Introduction
After a protracted battle, humans have triumphantly changed the course of nature and created social economics. In the meantime, environmental destruction has posed a realistic threat to mankind’s existence and progression. Water is the source of life. Oceans cover about 70 percent of the Earth. However, the freshwater resources that can be directly used by people contain less than 1% of the total water in the world. It is worth noting that the quality of available freshwater resources for humans is deteriorating due to demographic and environmental factors. [1,2,3][1][2][3]. At present, every year in some poor and backward countries, about 2 million people suffer from related diseases and eventually die because of a lack of access to clean water [4].
In water, most of the pollutants can be divided into heavy metal ions, acid ions, organic dyes, organic poisons, and drugs, which, to a certain extent, will cause allergy symptoms, nausea, headaches, heart disease, and other symptoms, and even lead to death [5]. A number of strategies have been used to treat these pollutants. They include nanofiltration [6], solvent extraction [7,8][7][8], resin use [9], photocatalytic degradation [10[10][11],11], cooling, ion exchange, chemical precipitation, electrochemical deposition, reverse osmosis, biological treatment [12[12][13][14][15],13,14,15], and others. However, most of these technologies have certain shortcomings, such as expensive equipment, complicated operations, and a long experimental period. Fortunately, adsorption, as one of the most promising pollutant treatment methods, has attracted the research interest of many researchers.
For the sake of adsorbing pollutants in water, it is necessary to choose the right material. Metal-organic frame materials were first proposed in 1999 by Omar Yaghi and his team at the University of California, Berkeley [16]. The material has the advantages of low density, a large specific surface area [17], ideal porosity [18[18][19],19], big pore size, while being adjustable [20,21,22,23][20][21][22][23]. Therefore, it presents an attractive topic in the fields of catalytic conversion [24,25,26[24][25][26][27],27], gas storage [28[28][29][30],29,30], electrochemical biosensing [31,32[31][32][33],33], drug slow-release control [34], molecular recognition [35[35][36][37],36,37], magnetism [38], adsorption and separation of pollutants [39[39][40][41],40,41], and so on. In order to fully exploit the advantages of MOFs in water purification, researchers modified the functional groups on MOFs so as to further boost the adsorption performance of MOFs. All these MOFs have achieved remarkable results in water purification.
2. Application of MOFs by Modified Functional Groups in Water Purification
2.1. N-Containing Group
2.1.1. -NH2
Liu et al. synthesized UiO-66-NH2-CS aerogel monomers with hierarchical structure by covalent cross-linking, which showed effective and stable adsorption of Pb2+ [83][42]. The aerogel was characterized by many pores and a low density. The adsorption of Pb2+ by the material was 102.03 mg·g−1. Among them, N and Pb2+ play a coordination role, and O plays a synergistic adsorption role to a certain extent. After three cycles, the material’s adsorption capacity remained at 90.12%. This strategy provides an effective and universal approach for using MOFs in the field of pollutant treatment (Figure 1).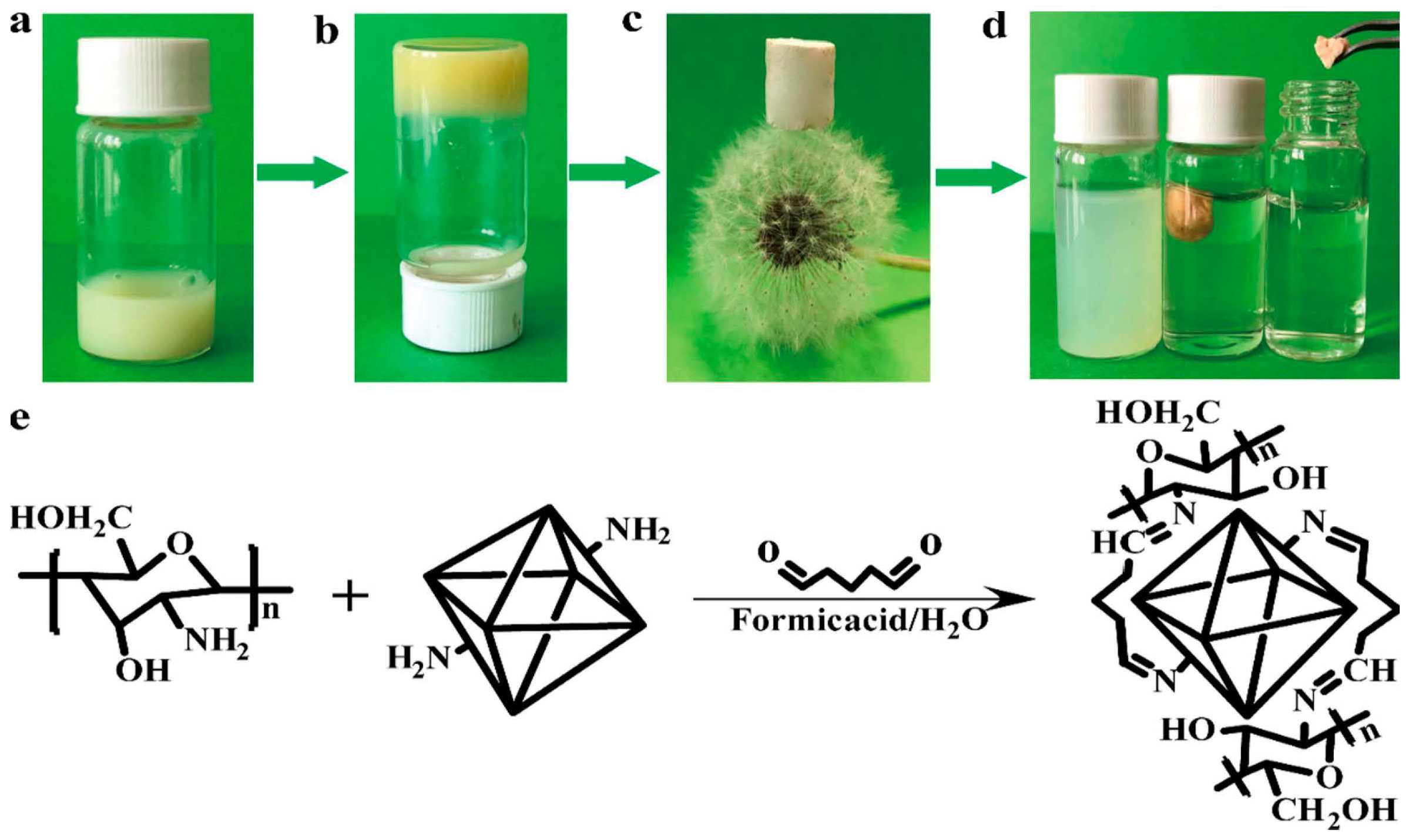
Figure 1. (a–c) Synthesis route of UiO-66-NH2-CS aerogel (d) Adsorption of Pb2+ by UiO-66-NH2-CS (e) Synthesis equation of UiO-66-NH2-CS.
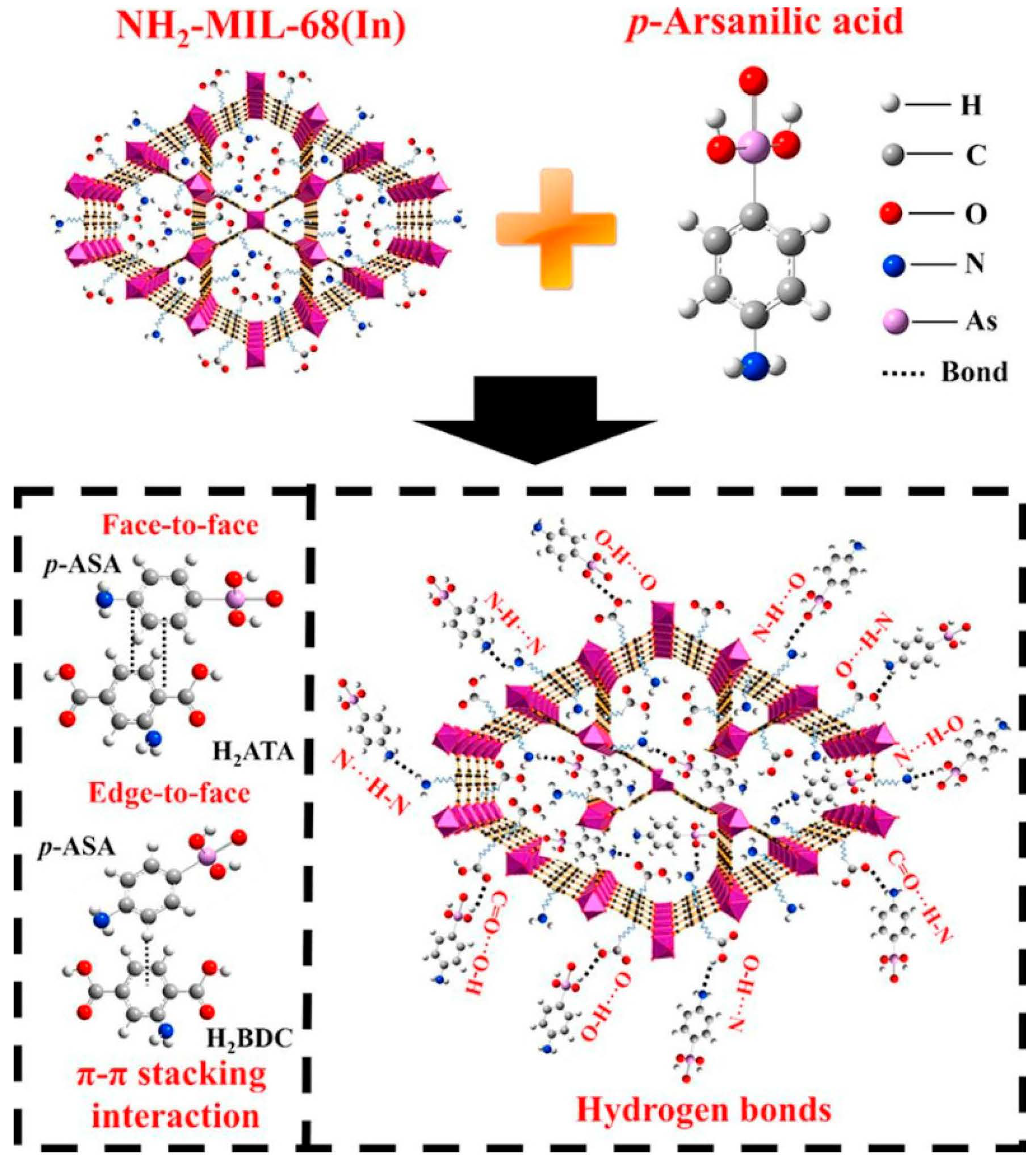
Figure 2. Structural formula of NH2-MIL-68(In) and main adsorption mechanism of p-ASA by NH2-MIL-68(In).
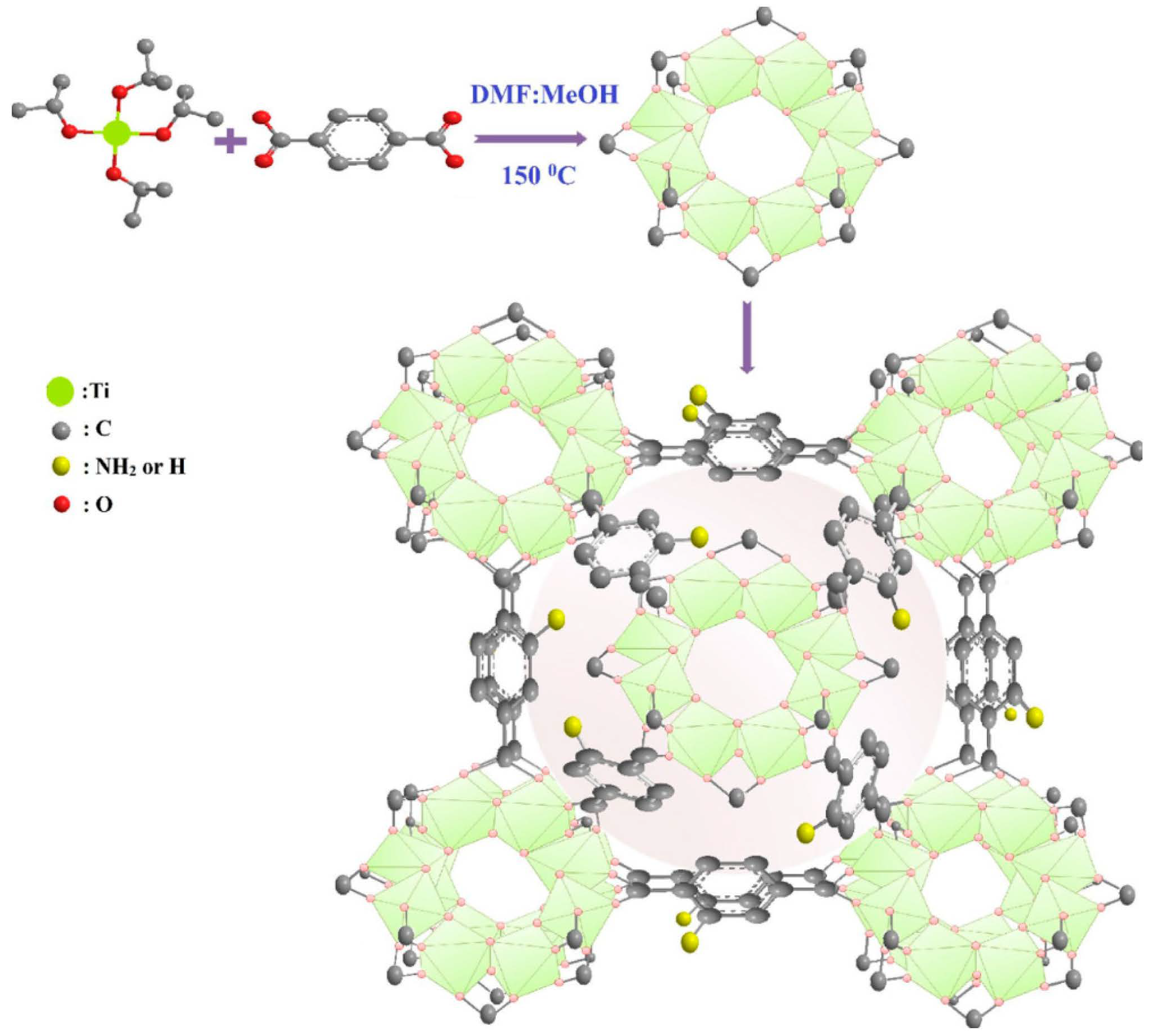
Figure 3. Synthesis and structure diagram of NH2-MIL-125(Ti).
2.1.2. -NMe3+
Wu et al. created a novel quaternary amine anion-exchange MOF (UiO-66-NMe3+) for the adsorption of 2,4-dichlorophenoxyacetic acid (2,4-d), a toxic pesticide that is extensively utilized [94][53]. UiO-66-NMe3+ had a maximum adsorption capacity for 2,4-d of 279 mg·g−1, which was much higher than UiO-66 and UiO-66-NH2. This is mainly attributed to the following two reasons. For one thing, the functionality of quaternary amine groups can effectively increase the electrostatic effect. For another, the π–π conjugation between 2,4-d and MOFs improves the adsorption properties of UiO-66-NMe3+. After seven cycles, the adsorption performance decreased only slightly. This technique offers the best alternative for effectively removing 2,4-d from complex water conditions by adsorption. Wei et al. created the functionalized quaternary ammonium salt MIL-101(Cr)-NMe3+ and took advantage of it as a novel adsorbent to wipe off diclofenac sodium (DCF) from wastewater [95][54]. The results showed that DCF quickly adsorbed onto MIL-101(Cr)-NMe3+. At 20 °C for 30 min, DCF can be adsorbed in large quantities (310.6 mg·g−1), which conforms to the pseudo-second-order kinetic model and the Langmuir adsorption. According to the adsorption mechanism analysis, the electrostatic interaction and π–π interaction between MIL-101(Cr)-NMe3+ and DCF are the reasons for the efficacious adsorption. Based on the results of the regeneration experiment, MIL-101(Cr)-NMe3+ could be recovered at least five times without suffering a substantial decrease in adsorption ability. Based on the preceding discussion, wresearchers can conclude that MOFs modified with -NH2 or -NMe3+ can be useful in water purification. For organic matter in water (such as organic poisons, dyes, and drugs), the general adsorption mechanism focuses on hydrogen bonding and electrostatic interaction. This is very understandable. Because N is a highly electronegative atom, it can be used as the acceptor of H, thus forming hydrogen bonds and realizing the adsorption of pollutants. For heavy metal ions in water, -NH2 successfully acts as a Lewis base. Because metal ions are Lewis acids, they can interact with -NH2 in Lewis acid–base reactions to produce the adsorption effect.2.2. S-Containing Group
2.2.1. -SH
Ke et al. selected the classic 3D Cu-MOF (HKUST-1) and used a simple post-synthesis strategy to achieve sulfhydryl functionalization of MOFs to investigate their application in removing Hg2+ from water [96][55]. They prepared a sequence of sulfhydryl-modified HKUST-1 by ligand-bonding the coordination unsaturated metal center in HKUST-1 to the -SH group in disulfide glycol. Under the same conditions, sulfhydryl-functionalized HKUST-1 showed very high adsorption capacity (714.29 mg·g−1) and fine adsorption affinity for Hg2+ in water. Nevertheless, unfunctionalized HKUST-1 demonstrated no adsorption for Hg2+. This method is considered to be a new and effective method for the treatment of heavy metal ions in water. Li et al. created sulfhydryl-modified MOFs (UiO-66-SH) by a simple tactic and used them for selectively extracting Hg2+ in aqueous solutions [97][56]. After the introduction of -SH, the rate of UiO-66-SH’s adsorption to Hg2+ was extremely rapid, showing high adsorption performance (3.91 mmol·g−1) in a broad pH range (2.3–8.0), and the adsorption efficiency remained high (> 90%) after seven regenerations. Even though there are other heavy metal ions (Cu2+, Mn2+, Ni2+, Cd2+, Ba2+, and Co2+), the adsorbent exhibited selective adsorption of Hg2+ (Figure 4). Density functional theory (DFT) calculations revealed that this was primarily due to the strong coordination between Hg2+ and -SH. It is worth mentioning that UiO-66-SH possesses a unique attraction for organic mercury forms as well. (PhHg+, EtHg+, and MeHg+). This method can provide a new idea for removing mercury from ecological environments.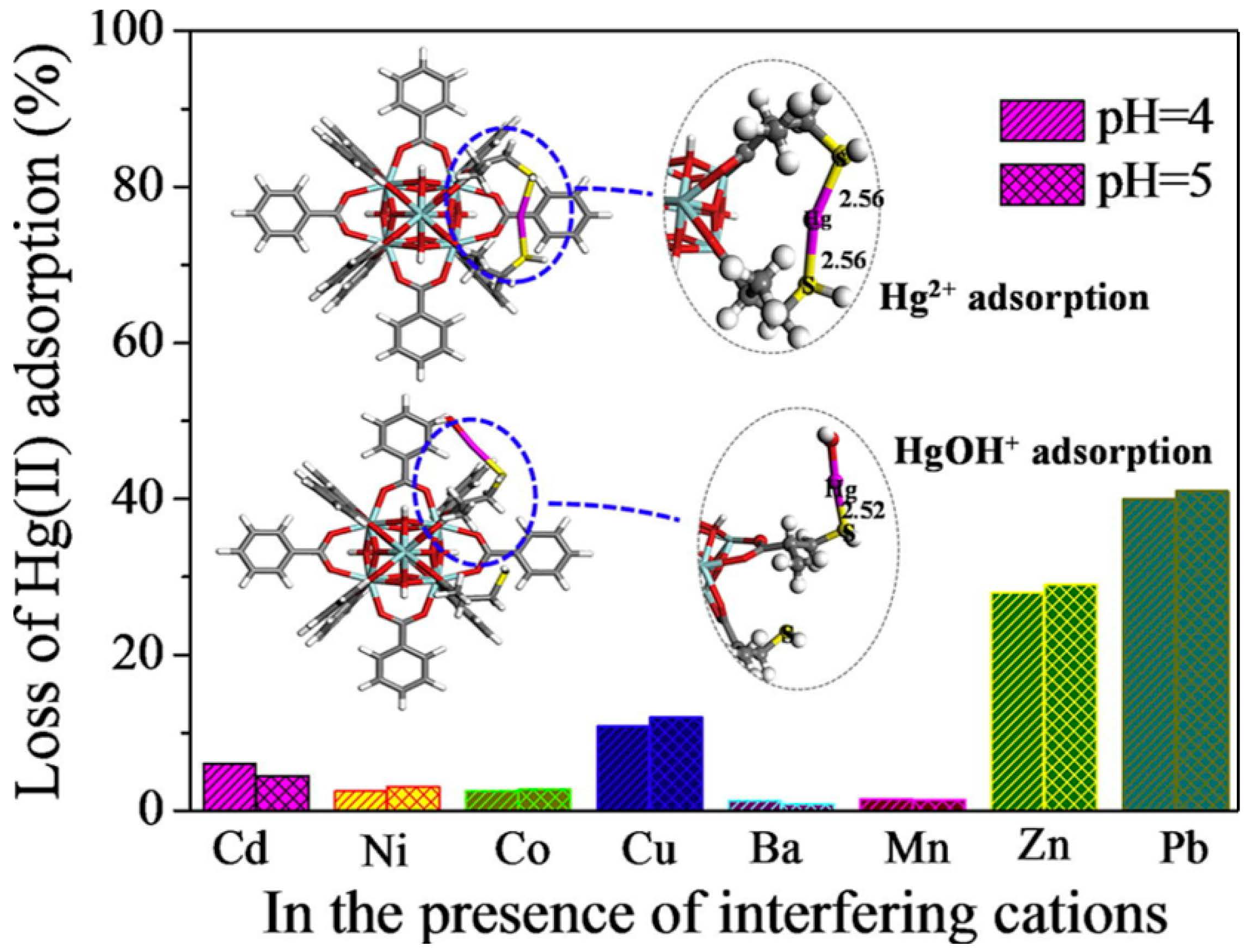
Figure 4. Schematic diagram of UiO-66-SH selective adsorption of Hg2+ at different pH values.
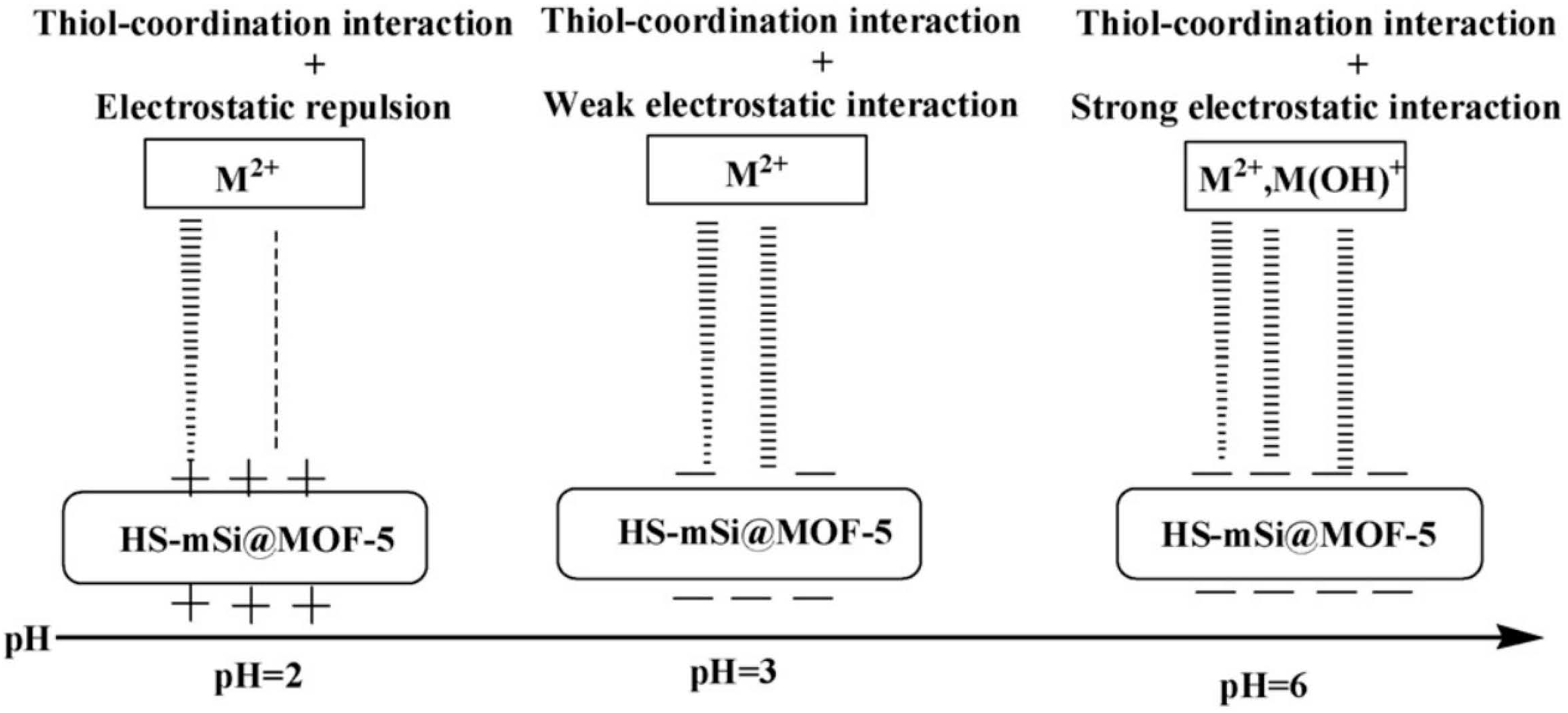
Figure 5. Schematic diagram of the strength of HS-mSi@MOF-5 on metal ions at different pH values.
2.2.2. -SO3H
Moradi et al. synthesized and used sulfonated MOFs supported on Fe3O4 NPs as a Fenton-like catalyst to remove methyl orange (MO) from water [100][59]. When the initial concentration is 100 mg·L−1, the incipient concentration of H2O2 is 40 mg·L−1, the microwave power is 500 W, and the pH value is 3.0, the rate of the elimination of MO is very fast. Only when the microwave radiation time is 6 min, as much as 99.9% of MO can be removed. Finally, microwave-induced Fe3O4@MIL-100(Fe)-SO3H degradation will be a wastewater dye removal technique with great promise. Hasan et al. first synthesized three Zr-MOF series materials (UiO-66-SO3H, UiO-66-NH2, and UiO-66) for the removal and adsorption of diclofenac sodium (DCF) in water and contrasted these three materials with activated carbon (AC) [101][60]. These three materials showed better adsorption performance than AC. The adsorption kinetics between UiO-66 and DCF are faster due to possible π–π interaction and electrostatic interaction. After the introduction of -SO3H, the kinetics and capacity (263 mg·g−1) of adsorption were significantly enhanced, which may result from the acid–base attraction of -SO3H in UiO-66-SO3H and -NH2 in DCF. Interestingly, however, the opposite trend was observed when UiO-66-NH2 was utilized as a sorbent. This is primarily due to the alkali-base rejection of -NH2 in DCF and UiO-66-NH2, on account of relevant results. Wang et al. immobilized -SO3H on the surface of MOFs to synthesize sulfonic acid-functionalized MOFs to remove Cd2+ from aqueous solutions [102][61]. Cu3(BTC)2-SO3H showed a relatively good absorption capacity of 88.7 mg·g−1, exceeding that of the reference adsorbent. Additionally, it has quick kinetics for the adsorption of Cd2+ from aqueous solutions that are 1–3 orders of magnitude higher than those of current adsorbent materials. Furthermore, despite the existence of other interfering ions, it indicates a strong selectivity for Cd2+ and is simple to regenerate and recycle without suffering a major reduction in Cd2+ adsorption capacity. Liu et al. focused on the adsorption behavior of UiO-66@mSi-SO3H, UiO-66@mSi-SH, and UiO-66 on Cd2+ [103][62]. In consideration of the data of the Elovich and the Langmuir models, the adsorption capacity can be UiO-66 < UiO-66@mSi-SH < UiO-66@mSi-SO3H. The theoretical maximum adsorption capacities of UiO-66@mSi-SO3H and UiO-66@mSi-SH for Cd2+ are 409.96 and 212 mg·g−1, respectively. In the presence of -SH and -SO3H, Cd2+ in water was replaced by adsorbents to accomplish the aim of getting rid of Cd2+. -SO3H has diverse and complex coordination forms, allowing UiO-66-mSi-SO3H to have an excellent adsorption capacity for removing Cd2+. Lv et al. synthesized a late-model magnetic nanocomposite with a core-shell structure of Fe3O4@UiO-66-SO3H by means of a viable stepwise assembly tactic and then made the most of it as an adsorbent to wipe off methylene blue (MB) from aqueous solutions [104][63]. A considerable affinity for MB may be shown in the synthesized Fe3O4@UiO-66-SO3H, which has a maximum adsorption capacity of 297.3 mg·g−1. The adsorption process is basically completed within 15 min. Because of the effect of charge selectivity, Fe3O4@UiO-66-SO3H has good reusability and can selectively adsorb MB from the mixed solution. The π–π interaction and electrostatic interaction are important during the adsorption process. (Figure 6).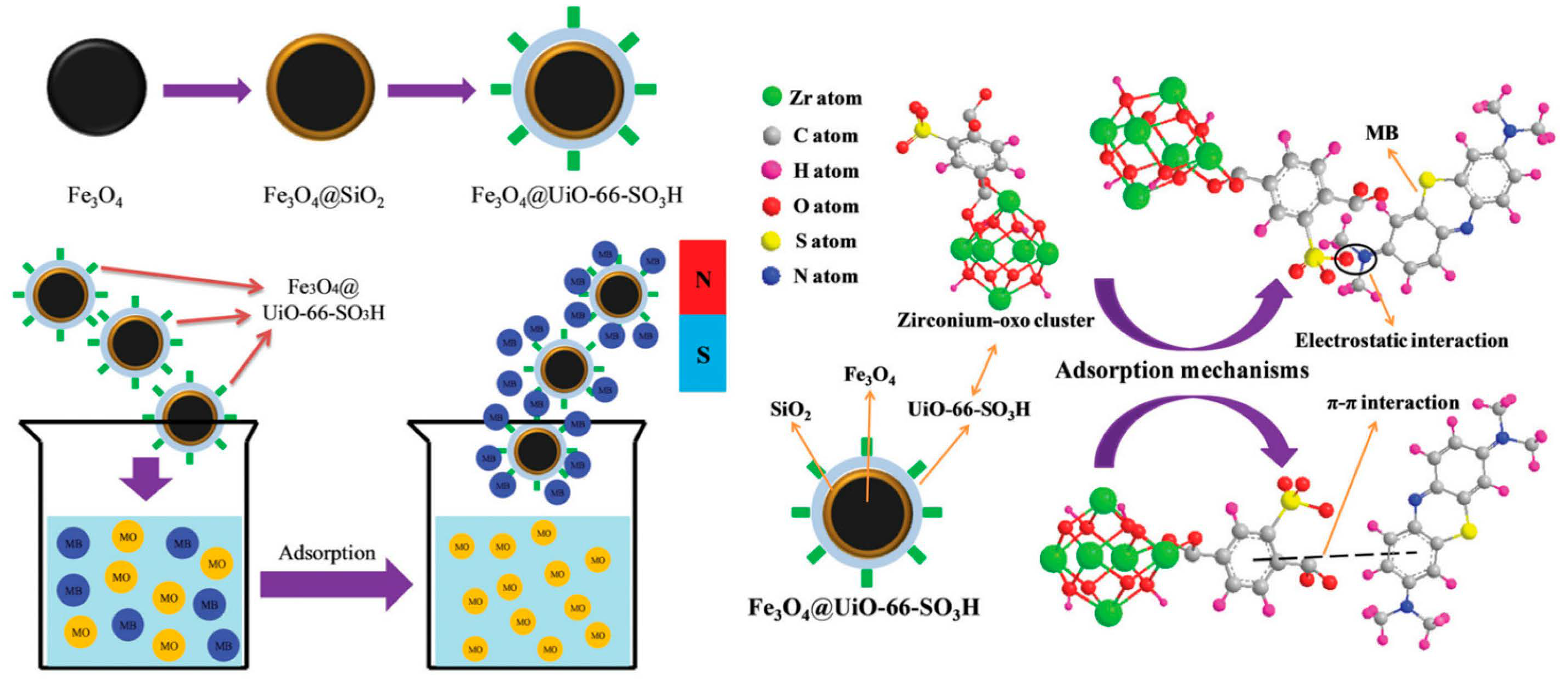
Figure 6. Schematic diagram of selective adsorption of MB by Fe3O4@UiO-66-SO3H and corresponding adsorption mechanism.
2.2.3. -SO4
Kang et al. selected Zr-BTC-NH2-SO4 as an adsorbent to capture radioactive Ba2+ in nuclear wastewater [105][64] (Figure 7). By reason of the plentiful -SO4 groups in the skeleton, which are strong Ba2+ chelating groups, and the fact that the binding site is completely exposed, in comparison to most adsorbents, Zr-BTC-NH2-SO4 has a greater adsorption capacity of 181.8 mg·g−1. When the concentration of interfering ions is ten times that of Ba2+, the material still shows excellent selectivity. Most crucially, the course of adsorption is irreversible for Ba2+, which can availably avert secondary pollution. This work has contributed to the development of adsorbents for the handling of radioactive Ba2+ from nuclear effluent.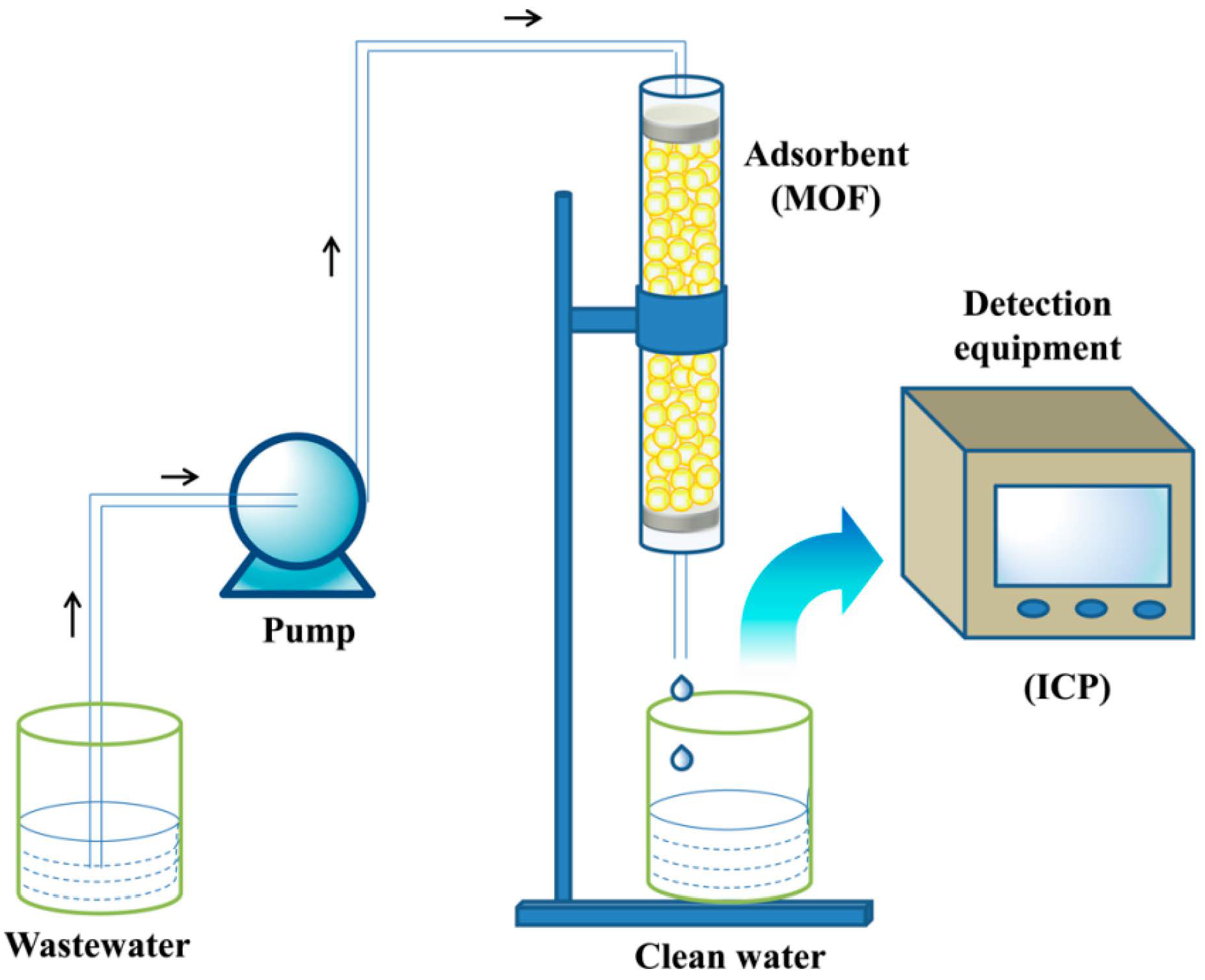
Figure 7. Schematic diagram of experimental setup used for breakthrough experiments.
References
- Johnson, N.; Gevenga, C.; Echeverria, J. Managing Water for People and Nature. Science 2001, 292, 1071–1072.
- Vorosmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global Water Resources: Vulnerability from Climate Change and Population Growth. Science 2000, 289, 284–288.
- Rolston, A. Social changes affect water quality too. Nature 2016, 536, 396.
- Kumar, P.; Bansal, V.; Kim, K.H.; Kwon, E.E. Metal-organic frameworks (MOFs) as futuristic options for wastewater treatment. J. Ind. Eng. Chem. 2018, 62, 130–145.
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310.
- Nthunya, L.N.; Bopape, M.F.; Mahlangu, O.T.; Mamba, B.B.; Van der Bruggen, B.; Quist-Jensen, C.A.; Richards, H. Fouling, performance and cost analysis of membrane-based water desalination technologies: A critical review. J. Environ. Manag. 2022, 301, 113922.
- Lv, Z.; Liang, C.; Cui, J.; Zhang, Y.; Xu, S. A facile route for the synthesis of mesoporous melamine-formaldehyde resins for hexavalent chromium removal. RSC Adv. 2015, 5, 18213–18217.
- Li, L.L.; Feng, X.Q.; Han, R.P.; Zang, S.Q.; Yang, G. Cr(VI) removal via anion exchange on a silver-triazolate MOF. J. Hazard. Mater. 2017, 321, 622–628.
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J. Hazard. Mater. 2015, 287, 364–372.
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418.
- Tanhaei, M.; Mahjoub, A.R.; Safarifard, V. Energy-efficient sonochemical approach for the preparation of nanohybrid composites from graphene oxide and metal-organic framework. Inorg. Chem. Commun. 2019, 102, 185–191.
- Shayegan, H.; Ali, G.A.M.; Safarifard, V. Amide-Functionalized Metal-Organic Framework for High Efficiency and Fast Removal of Pb(II) from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3170–3178.
- Sadegh, H.; Ali, G.A.M.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-ghoshekandi, R.; Nadagouda, M.N.; Sillanpaa, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostruct. Chem. 2017, 7, 1–14.
- Nthunya, L.; Masheane, M.; George, M.; Kime, M.-B.; Mhlanga, S. Removal of Fe and Mn From Polluted Water Sources in Lesotho Using Modified Clays. J. Water Chem. Technol. 2019, 41, 81–86.
- Makgabutlane, B.; Nthunya, L.N.; Musyoka, N.; Dladla, B.S.; Nxumalo, E.N.; Mhlanga, S.D. Microwave-assisted synthesis of coal fly ash-based zeolites for removal of ammonium from urine. RSC Adv. 2020, 10, 2416–2427.
- Furukawa, H.; Cordova, K.E.; O’ keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 974–987.
- Kennedy, R.D.; Krungleviciute, V.; Clingerman, D.J.; Mondloch, J.E.; Peng, Y.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Hupp, J.T.; Yildirim, T.; et al. Carborane-Based Metal-Organic Framework with High Methane and Hydrogen Storage Capacities. Chem. Mater. 2013, 25, 3539–3543.
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’ keeffe, M.; Kim, J.; Yaghi, O.M. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424–428.
- Slater, A.G.; Cooper, A.I. Function-led design of new porous materials. Science 2015, 348, 988–997.
- Chen, J.W.; Li, K.; Yang, J.; Gu, J.L. Hierarchical large-pore MOFs templated from poly(ethylene oxide)-b-polystyrene diblock copolymer with tuneable pore sizes. Chem. Commun. 2022, 58, 10028–10031.
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’ keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472.
- Cui, X.L.; Chen, K.J.; Xing, H.B.; Yang, Q.W.; Krishna, R.; Bao, Z.B.; Wu, H.; Zhou, W.; Dong, X.L.; Han, Y.; et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 2016, 353, 141–144.
- Hwang, Y.K.; Hong, D.Y.; Chang, J.S.; Jhung, S.H.; Seo, Y.K.; Kim, J.; Vimont, A.; Daturi, M.; Serre, C.; Férey, G. Amine Grafting on Coordinatively Unsaturated Metal Centers of MOFs: Consequences for Catalysis and Metal Encapsulation. Angew. Chem. Int. Edit. 2010, 47, 4144–4148.
- Liu, M.H. Precise Construction of MOFs with Catalytic Functions: Selective Regulators for Hydrogenation Reactions. Acta Phys.-Chim. Sin. 2016, 32, 2645–2646.
- Ji, P.F.; Manna, K.; Lin, Z.K.; Feng, X.Y.; Urban, A.; Song, Y.; Lin, W.B. Single-Site Cobalt Catalysts at New Zr12(μ3-O)8(μ3-OH)8(μ2-OH)6 Metal-Organic Framework Nodes for Highly Active Hydrogenation of Nitroarenes, Nitriles, and Isocyanides. J. Am. Chem. Soc. 2017, 12, 12234–12242.
- Lai, Q.; Zheng, L.R.; Liang, Y.Y.; He, J.P.; Zhao, J.X.; Chen, J.H. Metal-Organic-Framework-Derived Fe-N/C Electrocatalyst with Five-Coordinated Fe-Nx Sites for Advanced Oxygen Reduction in Acid Media. ACS Catal. 2017, 7, 1655–1663.
- Shen, J.Q.; Liao, P.Q.; Zhou, D.D.; He, C.T.; Wu, J.X.; Zhang, W.X.; Zhang, J.P.; Chen, X.M. Modular and Stepwise Synthesis of a Hybrid Metal-Organic Framework for Efficient Electrocatalytic Oxygen Evolution. J. Am. Chem. Soc. 2017, 139, 1778–1781.
- Qasem, N.A.A.; Ben-Mansour, R.; Habib, M.A. Efficient CO2 adsorptive storage using MOF-5 and MOF-177. Appl. Energ. 2017, 210, 317–326.
- Zou, L.F.; Zhou, H.C. Hydrogen Storage in Metal-Organic Frameworks. In Nanostructured Materials for Next-Generation Energy Storage and Conversion; Chen, Y.P., Bashir, S., Liu, J.L., Eds.; Springer: Berlin, Heidelberg, Germany, 2017; Volume 22, pp. 143–170.
- Liu, D.M.; Wu, H.H.; Wang, S.Z.; Xie, Z.G.; Li, J.; Lin, W.B. A high connectivity metal-organic framework with exceptional hydrogen and methane uptake capacities. Chem. Sci. 2012, 3, 3032–3037.
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Duyne, R.P.V.; Hupp, J.T. Metal-Organic Framework Materials as Chemical Sensors. Chem. Rev. 2015, 112, 1105–1125.
- Chen, B.; Yang, Y.; Zapata, F.; Lin, G.; Qian, G.; Lobkovsky, E.B. Luminescent Open Metal Sites within a Metal-Organic Framework for Sensing Small Molecules. Adv. Mater. 2010, 19, 1693–1696.
- Liu, T.Z.; Rong, H.; Zhang, X.; Zhang, K.L.; Liu, Y.; Zhang, X.B.; Bai, R.Y.; Li, D.; Yang, Y.H. Metal-Organic Framework Nanomaterials as Novel Signal Probes for Electron Transfer Mediated Ultrasensitive Electrochemical Immunoassay. Anal. Chem. 2016, 88, 12516–12523.
- Chen, Y.J.; Li, P.; Modica, J.A.; Drout, R.J.; Farha, O.K. Acid-Resistant Mesoporous Metal-Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release. J. Am. Chem. Soc. 2018, 140, 5678–5681.
- Cychosz, K.A.; Wong-Foy, A.G.; Matzger, A.J. Liquid Phase Adsorption by Microporous Coordination Polymers: Removal of Organosulfur Compounds. J. Am. Chem. Soc. 2008, 130, 6938–6939.
- Herm, Z.R.; Wiers, B.M.; Mason, J.A.; Baten, J.M.V.; Hudson, M.R.; Zajdel, P.; Brown, C.R.; Masciocchi, N.; Krishna, R.; Long, J.R. Separation of Hexane Isomers in a Metal-Organic Framework with Triangular Channels. Science 2013, 340, 960–964.
- Das, S.; Xu, S.X.; Ben, T.; Qiu, S.L. Chiral Recognition and Separation by Chirality-Enriched Metal-Mrganic Frameworks. Angew. Chem. Int. Edit. 2018, 57, 8629–8633.
- Ryu, D.W.; Lee, W.R.; Lee, J.W.; Yoon, J.H.; Kim, H.C.; Koh, E.K.; Hong, C.S. Magnetic metal-organic framework constructed from a paramagnetic metalloligand exhibiting a significant sorption and reversible magnetic conversions. Chem. Commun. 2009, 46, 8779–8781.
- Lin, Y.C.; Kong, C.L.; Zhang, Q.J.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296.
- Murray, L.J.; Dincǎ, M.; Long, J.R. Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314.
- Yang, Q.Y.; Ma, L.L.; Zhong, C.L.; An, X.H.; Liu, D.H. Enhancement of CO2/N2 Mixture Separation Using the Thermodynamic Stepped Behavior of Adsorption in Metal-Organic Frameworks. J. Phys. Chem. C 2011, 115, 2790–2797.
- Liu, Q.; Li, S.S.; Yu, H.H.; Zeng, F.M.; Li, X.; Su, Z.M. Covalently crosslinked zirconium-based metal-organic framework aerogel monolith with ultralow-density and highly efficient Pb(II) removal. J. Colloid Interface Sci. 2020, 561, 211–219.
- Azmi, L.H.M.; Williams, D.R.; Ladewig, B.P. Polymer-Assisted Modification of Metal-Organic Framework MIL-96(Al): Influence on Particle Size, Crystal Morphology and Perfluorooctanoic Acid (PFOA) Removal. Chemosphere 2020, 262, 128072.
- Samuel, M.S.; Savunthari, K.V.; Chandrasekar, N.; Balaji, R.; Selvarajan, E. Removal of environmental contaminants of emerging concern using metal-organic framework composite. Environ. Technol. Innov. 2022, 25, 102216.
- Park, J.M.; Jhung, S.H. A remarkable adsorbent for removal of bisphenol S from water: Aminated metal-organic framework, MIL-101-NH2. Chem. Eng. J. 2020, 396, 125224.
- Wu, S.; Ge, Y.; Wang, Y.; Chen, X.; Li, F.; Xuan, H.; Li, X. Adsorption of Cr (VI) on nano UiO-66-NH2 MOFs in water. Environ. Technol. 2018, 39, 1937–1948.
- Roushani, M.; Saedi, Z.; Baghelani, Y.M. Removal of cadmium ions from aqueous solutions using TMU-16-NH2 metal organic framework. Environ. Nanotechnol. Monit. Manag. 2017, 7, 89–96.
- Lv, Y.C.; Zhang, R.S.; Zeng, S.L.; Liu, K.Y.; Huang, S.Y.; Liu, Y.F.; Xu, P.F.; Lin, C.X.; Cheng, Y.J.; Liu, M.H. Removal of p-arsanilic acid by an amino-functionalized indium-based metal-organic framework: Adsorption behavior and synergetic mechanism. Chem. Eng. J. 2018, 339, 359–368.
- Roushani, M.; Saedi, Z.; Beygi, T.M. Anionic dyes removal from aqueous solution using TMU-16 and TMU-16-NH2 as isoreticular nanoporous metal organic frameworks. J. Taiwan Inst. Chem. Eng. 2016, 66, 164–171.
- Haque, E.; Lo, V.; Minett, A.I.; Harris, A.T.; Church, T.L. Dichotomous adsorption behaviour of dyes on an amino-functionalised metal-organic framework, amino-MIL-101(Al). J. Mater. Chem. A 2014, 2, 193.
- Liu, H.C.; Chen, L.G.; Ding, J. Adsorption behavior of magnetic amino-functionalized metal-organic framework for cationic and anionic dyes from aqueous solution. RSC Adv. 2016, 6, 48884.
- Oveisi, M.; Asli, M.A.; Mahmoodi, N.M. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: Synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J. Hazard. Mater. 2018, 347, 123–140.
- Wu, G.G.; Ma, J.P.; Li, S.; Wang, S.S.; Jiang, B.; Luo, S.Y.; Li, J.H.; Wang, X.Y.; Guan, Y.F.; Chen, L.X. Cationic metal-organic frameworks as an efficient adsorbent for the removal of 2,4-dichlorophenoxyacetic acid from aqueous solutions. Environ. Res. 2020, 186, 109542.
- Wei, Y.; Wei, C.; Xia, Y. Efficient adsorption and removal of diclofenac sodium from water with quaternary ammonium functionalized metal-organic frameworks. Chin. J. Chromatogr. 2018, 36, 222–229.
- Ke, F.; Qiu, L.G.; Yuan, Y.P.; Peng, F.M.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2011, 196, 36–43.
- Li, J.; Liu, Y.; Ai, Y.J.; Alsaedi, A.; Hayat, T.; Wang, X.K. Combined experimental and theoretical investigation on selective removal of mercury ions by metal organic frameworks modified with thiol groups. Chem. Eng. J. 2018, 354, 790–801.
- Singh, N.; Srivastava, I.; Dwivedi, J.; Sankararamakrishnan, L. Ultrafast removal of ppb levels of Hg(II) and volatile Hg(0) using post modified metal organic framework. Chemosphere 2020, 270, 129490.
- Zhang, J.M.; Xiong, Z.H.; Li, C.; Wu, C.S. Exploring a thiol-functionalized MOF for elimination of lead and cadmium from aqueous solution. J. Mol. Liq. 2016, 221, 43–50.
- Moradi, S.E.; Dadfarnia, S.; Shabani, A.M.H.; Emami, S. Microwave-enhanced Fenton-like degradation by surface-modified metal-organic frameworks as a promising method for removal of dye from aqueous samples. Turk. J. Chem. 2017, 41, 426–439.
- Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive removal of diclofenac sodium from water with Zr-based metal-organic frameworks. Chem. Eng. J. 2016, 284, 1406–1413.
- Wang, Y.; Ye, G.Q.; Chen, H.H.; Hu, X.Y.; Niu, Z.; Ma, S.Q. Functionalized metal-organic framework as a new platform for efficient and selective removal of cadmium(II) from aqueous solution. J. Mater. Chem. A 2015, 3, 15292–15298.
- Liu, J.M.; Cui, H.H.; Li, J.H.; Chen, M.C. A research on the cadmium ions adsorption of Sulfhydryl- and sulfo-functionalized UiO-66 with silica layer from water. J. Environ. Chem. Eng. 2021, 9, 104621.
- Lv, S.W.; Liu, J.M.; Li, C.Y.; Ma, H.; Wang, Z.H.; Zhao, N.; Wang, S. Fabrication of Fe3O4@UiO-66-SO3H core-shell functional adsorbents for highly selective and efficient removal of organic dyes. New J. Chem. 2019, 43, 7770.
- Kang, C.F.; Peng, Y.G.; Tang, Y.Z.; Huang, H.L.; Zhong, C.L. Sulfate-Rich Metal-Organic Framework for High Efficiency and Selective Removal of Barium from Nuclear Wastewater. Ind. Eng. Chem. Res. 2017, 56, 13866–13873.
- Peng, Y.G.; Huang, H.L.; Liu, D.H.; Zhong, C.L. Radioactive Barium Ion Trap Based on Metal-Organic Framework for Efficient and Irreversible Removal of Barium from Nuclear Wastewater. ACS Appl. Mater. Interfaces 2016, 8, 8527–8535.
- Chen, J.; Li, K.; Chen, L.; Liu, R.; Huang, X.; Ye, D. Conversion of fructose into 5-hydroxymethylfurfural catalyzed by recyclable sulfonic acid-functionalized metal-organic frameworks. Green Chem. 2014, 16, 2490–2499.
More
