Selenium (Se) is an essential trace element for humans and animals. Biofortification is a quick, efficient, and sustainable way to lower micronutrient deficiencies. The strategies of biofortification are the approaches employed to achieve biofortification. Plants and animal produce are classified as biofortified when there is an increase in the Se content in the edible portions of plants and food animals.
- selenium
- biofortification
- plants
- livestock
1. Introduction
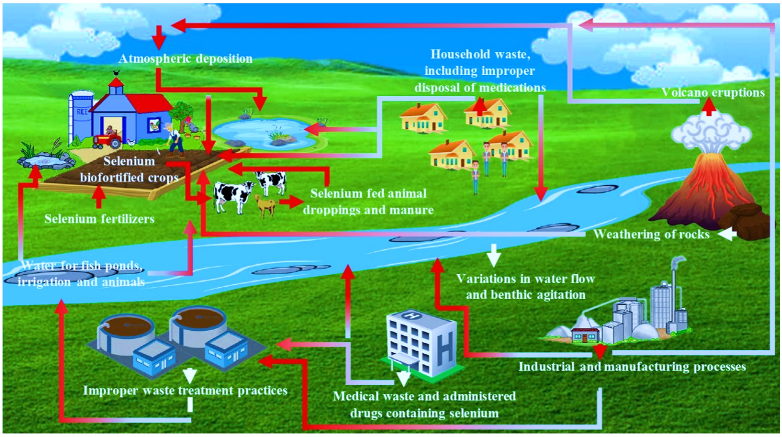
2. Se Biofortification Strategies
2.1. Se Forms
2.2. Se Biofortification Strategies in Plants
2. Se Biofortification Strategies
2.1. Se Forms
2.2. Se Biofortification Strategies in Plants
2.2.1. Foliar Application
Foliar application appears to be the most popular method of applying selenium among all methods because of its simplicity and preferable outcomes. The danger of environmental contamination also seems to be lower. Studies have demonstrated that foliar spray entails minimal use of Se salts [29][30]. This technique entails spraying a crop’s leaf surface with a Se-containing solution. Selenium enters the plant through the leaf cuticles. Particles can also enter plants through trichomes, stomata, stigma, and hydathodes [31]. In this respect, soil chemistry and microbiological processes have less of an impact on Se, resulting in a higher absorption rate with modest quantities of administered Se solution. With this strategy, there are changes in plant-specific parameters that must be taken into account, including the quantity of Se applied, leaf area and surface structure, and leaf structure.
2.2.2. Soil Application
2.2.1. Foliar Application
Foliar application appears to be the most popular method of applying selenium among all methods because of its simplicity and preferable outcomes. The danger of environmental contamination also seems to be lower. Studies have demonstrated that foliar spray entails minimal use of Se salts [63,64]. This technique entails spraying a crop’s leaf surface with a Se-containing solution. Selenium enters the plant through the leaf cuticles. Particles can also enter plants through trichomes, stomata, stigma, and hydathodes [65]. In this respect, soil chemistry and microbiological processes have less of an impact on Se, resulting in a higher absorption rate with modest quantities of administered Se solution. With this strategy, there are changes in plant-specific parameters that must be taken into account, including the quantity of Se applied, leaf area and surface structure, and leaf structure.
2.2.2. Soil Application
2.2.3. Microbial-Assisted Biofortification
2.2.4. Genetic Biofortification
Genetic engineering has the capability to enhance the capacity of plants to accumulate selenium as an alternative to agronomic approaches. Nevertheless, due to the stringent limitations on the usage of transgenics that are still present in several nations, genetic engineering is still not as prevalent and recognized as agronomic biofortification [94][46]. However, various chromosomal loci linked to elevated Se accumulation in a number of crops have been reported [74,95,96][36][47][48]. Marker-assisted breeding can be employed to transfer high-Se chromosomal loci from high-yielding, low-Se edible plant varieties into the breeding population [75][37].2.2.5. Crop Breeding
Some researchers believe that conventional crop breeding may be a sustainable and long-term approach to crop biofortification with Se [101][49]. However, compared to genetic biofortification crop breeding is a slower and less accurate method as this procedure is generally executed by hand. For instance, one study lasted for about five years [102][50]. Additionally, establishing appropriate and viable genotypic variation may be difficult [103][51]. Nevertheless, it can be utilized to create new plant types with enhanced features. Crop breeding for Se biofortification uses the conventional procedure of cross-pollinating two separate plants to develop a new hybrid plant with a mix of features from both parents to promote Se absorption and translocation to edible portions of the crop. The researchers’ goal in the above-mentioned study was to breed Se-rich red glutinous rice and evaluate the concentration of Se and protein in various parts of the rice.2.3. Se Biofortification Strategies in Livestock
In livestock, biofortification comprises employing agronomic or biotechnological techniques to increase the quantity of vital nutrients in edible sections of animals [104][52]. Se fertilization of farmlands, dietary supplementation via feed concentrate rations, and direct administration, including injections, are viable supplementation techniques. High Se feed concentrations are expected to raise Se concentrations in livestock. Animals can generate a variety of selenoproteins, including glutathione peroxidase, selenoprotein P, selenoprotein W, thioredoxin reductase, and other iodothyronine deiodinases, using absorbed selenium forms [105][53]. However, excessive Se ingestion in livestock (5–50 mg per kg of mass) may cause alkali disease, characterized by hoof deformities, a lack of vitality, anemia, and stiffness [106][54].References
- Oldfield, J. Se World Atlas; Se-Tellurium Development Association (STDA): Grimbergen, Belgium, 1999.
- Garside, M. Production Volume of Selenium Worldwide in 2020, by Country. 2022. Available online: https://www.statista.com/statistics/1312522/selenium-production-volume-worldwide-by-country/#:~:text=The%20country%20with%20the%20largest,3.33%20thousand%20metric%20tons%20worldwide. (accessed on 13 December 2022).
- Asante-Badu, B.; Kgorutla, L.; Li, S.; Danso, P.; Xue, Z.; Qiang, G. Phytoremediation of organic and inorganic compounds in a natural and an agricultural environment: A review. Appl. Ecol. Environ. Res. 2020, 18, 6875–6904.
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383.
- Rodriguez, M.M.; Rivero, V.C.; Ballesta, R.J.; Rivero, M.V.C. Selenium Distribution in Topsoils and Plants of a Semi-arid Mediterranean Environment. Environ. Geochem. Health 2005, 27, 513–519.
- Ye, W.; Zhu, R.; Yuan, L.; Zhang, W.; Zang, H.; Jiao, Y.; Yin, X. The influence of sea animals on selenium distribution in tundra soils and lake sediments in maritime Antarctica. Chemosphere 2021, 291, 132748.
- Feinberg, A.; Stenke, A.; Peter, T.; Hinckley, E.L.S.; Driscoll, C.T.; Winkel, L.H. Reductions in the deposition of sulfur and selenium to agricultural soils pose risk of future nutrient deficiencies. Commun. Earth Environ. 2021, 2, 101.
- Ye, W.; Yuan, L.; Zhu, R.; Yin, X.; Bañuelos, G. Selenium volatilization from tundra soils in maritime Antarctica. Environ. Int. 2021, 146, 106189.
- Hoet, P. Sélénium et ses Composés; Elsevier Masson SAS: Paris, France, 2013.
- Hartikainen, H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elements Med. Biol. 2005, 18, 309–318.
- Dhillon, K.S.; Dhillon, S.K.; Singh, B. Genesis of seleniferous soils and associated animal and human health problems. Adv. Agron. 2018, 154, 1–80.
- Emmanuelle, B.; Virginie, M.; Fabienne, S.; Isabelle, I.; Martine, P.-G.; Bernard, L.; Sylvie, R. Selenium exposure in subjects living in areas with high selenium concentrated drinking water: Results of a French integrated exposure assessment survey. Environ. Int. 2012, 40, 155–161.
- Vinceti, M.; Crespi, C.M.; Bonvicini, F.; Malagoli, C.; Ferrante, M.; Marmiroli, S.; Stranges, S. The need for a reassessment of the safe upper limit of selenium in drinking water. Sci. Total. Environ. 2013, 443, 633–642.
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Sci. Total. Environ. 2015, 521–522, 246–260.
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228.
- Dungan, R.S.; Frankenberger, W.T. Microbial Transformations of Selenium and the Bioremediation of Seleniferous Environments. Bioremediation J. 1999, 3, 171–188.
- Besser, J.M.; Canfield, T.J.; La Point, T.W. Bioaccumulation of organic and inorganic selenium in a laboratory food chain. Environ. Toxicol. Chem. Int. J. 1993, 12, 57–72.
- Elrashidi, M.A.; Adriano, D.C.; Lindsay, W.L. Solubility, speciation, and transformations of selenium in soils. Selenium Agric. Environ. 1989, 23, 51–63.
- Freeman, J.L.; Bañuelos, G.S. Selection of Salt and Boron Tolerant Selenium Hyperaccumulator Stanleya pinnata Genotypes and Characterization of Se Phytoremediation from Agricultural Drainage Sediments. Environ. Sci. Technol. 2011, 45, 9703–9710.
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol. Plant. 2015, 37, 41.
- Mikkelsen, R.L.; Page, A.L.; Bingham, F.T.; Jacobs, L.W. Factors Affecting Selenium Accumulation by Agricultural Crops. Selenium Agric. Environ. 2015, 23, 65–94.
- Fernandez-Martinez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Bio/Technol. 2009, 8, 81–110.
- Masscheleyn, P.H.; Delaune, R.D.; Patrick, W.H. Arsenic and Selenium Chemistry as Affected by Sediment Redox Potential and pH. J. Environ. Qual. 1991, 20, 522–527.
- McNeal, J.M.; Balistrieri, L.S.; Jacobs, L.W. Geochemistry and Occurrence of Selenium: An Overview. Selenium Agric. Environ. 2015, 23, 1–13.
- Kikkert, J.; Berkelaar, E. Plant Uptake and Translocation of Inorganic and Organic Forms of Selenium. Arch. Environ. Contam. Toxicol. 2013, 65, 458–465.
- Qin, S.; Gao, J.; Huang, K. Effects of different selenium sources on tissue selenium concentrations, blood GSH-Px activities and plasma interleukin levels in finishing lambs. Biol. Trace Elem. Res. 2007, 116, 91–102.
- Kruzhel, B.; Bakowska, M.; Vovk, S.; Nowakowska, E.; Sergei, P. Selenium in the diet of ruminants. Acta Sci. Polonorum. Zootech. 2014, 13, 5–16.
- Lyons, M.P.; Papazyan, T.T.; Surai, P.F. Selenium in Food Chain and Animal Nutrition: Lessons from Nature -Review-. Asian-Australas. J. Anim. Sci. 2007, 20, 1135–1155.
- Hawrylak-Nowak, B.; Hasanuzzaman, M.; Matraszek-Gawron, R. Mechanisms of Selenium-Induced Enhancement of Abiotic Stress Tolerance in Plants. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 269–295.
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium Enrichment of Horticultural Crops. Molecules 2017, 22, 933.
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712.
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.-R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147.
- Ebrahimi, N.; Stoddard, F.L.; Hartikainen, H.; Seppänen, M.M. Plant species and growing season weather influence the efficiency of selenium biofortification. Nutr. Cycl. Agroecosystems 2019, 114, 111–124.
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074.
- Dongli, L.; Liang, D.; Qin, S.; Feng, P.; Wu, X. Effects of selenite and selenate application on growth and shoot selenium accumulation of pak choi (Brassica chinensis L.) during successive planting conditions. Environ. Sci. Pollut. Res. 2015, 22, 11076–11086.
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2015, 117, 217–235.
- Wu, Z.; Banuelos, G.S.; Lin, Z.Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136.
- Wan, Y.; Yu, Y.; Wang, Q.; Qiao, Y.; Li, H. Cadmium uptake dynamics and translocation in rice seedling: Influence of different forms of selenium. Ecotoxicol. Environ. Saf. 2016, 133, 127–134.
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium Cycling Across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239.
- Zhang, L.; Hu, B.; Li, W.; Che, R.; Deng, K.; Li, H.; Yu, F.; Ling, H.; Li, Y.; Chu, C. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol. 2014, 201, 1183–1191.
- Barret, M.; Morrissey, J.P.; O’Gara, F. Functional genomics analysis of plant growth-promoting rhizobacterial traits involved in rhizosphere competence. Biol. Fertil. Soils 2011, 47, 729–743.
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721.
- Fordyce, F. Selenium Geochemistry and Health. AMBIO 2007, 36, 94–97.
- Acuña, J.J.; Jorquera, M.A.; Barra, P.J.; Crowley, D.E.; Mora, M.D.L.L. Selenobacteria selected from the rhizosphere as a potential tool for Se biofortification of wheat crops. Biol. Fertil. Soils 2012, 49, 175–185.
- Yasin, M.; El Mehdawi, A.F.; Jahn, C.E.; Anwar, A.; Turner, M.F.S.; Faisal, M.; Pilon-Smits, E.A.H. Seleniferous soils as a source for production of selenium-enriched foods and potential of bacteria to enhance plant selenium uptake. Plant Soil 2014, 386, 385–394.
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84.
- Zhu, Y.-G.; Pilon-Smits, E.A.H.; Zhao, F.-J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 436–442.
- Ates, D.; Sever, T.; Aldemir, S.; Yagmur, B.; Temel, H.Y.; Kaya, H.B.; Tanyolac, B. Identification QTLs controlling genes for Se uptake in lentil seeds. PLoS ONE 2016, 11, e0149210.
- Liu, C.; Ding, S.; Zhang, A.; Hong, K.; Jiang, H.; Yang, S.; Ruan, B.; Zhang, B.; Dong, G.; Guo, L.; et al. Development of nutritious rice with high zinc/selenium and low cadmium in grains through QTL pyramiding. J. Integr. Plant Biol. 2020, 62, 349–359.
- Liang, Y.; Farooq, M.U.; Zeng, R.; Tang, Z.; Zhang, Y.; Zheng, T.; Zhu, J. Breeding of selenium rich red glutinous rice, protein extraction and analysis of the distribution of selenium in grain. Int. J. Agric. Biol. 2018, 20, 1005–1011.
- Mao, H.; Wang, J.; Wang, Z.; Zan, Y.; Lyons, G.; Zou, C. Using agronomic biofortification to boost zinc, selenium, and iodine concentrations of food crops grown on the loess plateau in China. J. Soil Sci. Plant Nutr. 2014, 14, 459–470.
- Yuan, L.; Yin, X.; Zhu, Y.; Li, F.; Huang, Y.; Liu, Y.; Lin, Z. Selenium in plants and soils, and selenosis in Enshi, China: Implications for selenium biofortification. Phytoremediation Biofortification Two Sides One Coin 2012, 7–31.
- Rayman, M.P. The use of high-selenium yeast to raise selenium status: How does it measure up? Br. J. Nutr. 2004, 92, 557–573.
- Jeong, D.; Lee, K.H. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: The selenium paradox (Review). Mol. Med. Rep. 2011, 5, 299–304.
