Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Estefan Monteiro Da Fonseca.
The particular characteristics of the plastic matrix, such as its floating ability and hydrophobicity, have created a new unique substratum for microbial colonization. The new micro-niche thus created becomes occupied by a specific biofilm called the plastisphere.
- biofouling
- hydrodynamics
- sediments
- plastics biodegradation
- plastisphere
1. The Plastisphere
The particular characteristics of the plastic matrix, such as its floating ability and hydrophobicity, have created a new unique substratum for microbial colonization [25,50,51][1][2][3]. The new micro-niche thus created becomes occupied by a specific biofilm called the plastisphere [25,42,52,53,54,55][1][4][5][6][7][8].
The total mass of the plastisphere in the oceans cannot be neglected, representing about 0.01–0.2% of the total microbial biomass in their surface waters [42][4]. However, because of the unknown total amount of plastic discarded in the oceans, the total mass of the plastisphere may be much higher than this [3,42][4][9]. Indeed, some authors have described MPs and their associated plastisphere as the eighth continent [52,56,57][5][10][11]. More research on the plastisphere and its importance in biogeochemical cycling and the resulting environmental balance [58][12] is fundamental.
As a result of the different physicochemical conditions in fresh and saline water, the microbiota in these two ecosystems is distinct, which can impact the structure and evolution of the microbial populations in these environments [53][6] The microbial ecology of the plastisphere, however, is mainly controlled by the composition of the colonized plastic [59][13]; MPs work as a filter for microorganisms in the environment.
As hydrophobic organic surfaces with large surface area:volume ratios, MPs readily attract organic matter from the water column, including organic carbon sources and pollutants such as pesticides [60][14] and hydrocarbons [61][15]. In addition, many of the chemical compounds added to plastics during their industrial production are toxic to the colonizing microorganisms. These characteristics turn the MP surface into a very complex substratum that is highly selective for colonization by specific microbial species.
Nowadays, thanks to new technologies based on metagenomics, it has been possible to observe the complexity and partially understand the operation of the plastisphere. Reisser et al. (2014) [62][16] and Dussud et al. (2018) [33][17] confirmed the influence of certain properties of plastic fragments such as composition, size, degree of degradation, and surface roughness. Amaral-Zettler et al. (2015) [42][4] noted important differences between the microorganisms colonizing MPs in two different oceans, and between planktonic and sessile cells on MPs in the same environment. Oberbeckmann et al. (2018) [2][18] and Debroas et al. (2017) [63][19] showed that the microbial communities present on the surfaces of marine MPs are very different from those in surrounding middle and upper waters or on other particle types (Figure 1). The authors reported greater abundance and richness of colonizing bacterial assemblages on a natural substrate compared with MP communities. This suggests that the modern universal availability of MPs in our oceans not only affects the structure, composition, and functional properties of attached bacteria but also represents a potential ecological risk as a function of the high stability, pathogenicity, and stress tolerance of the bacterial communities present on the MP surface.
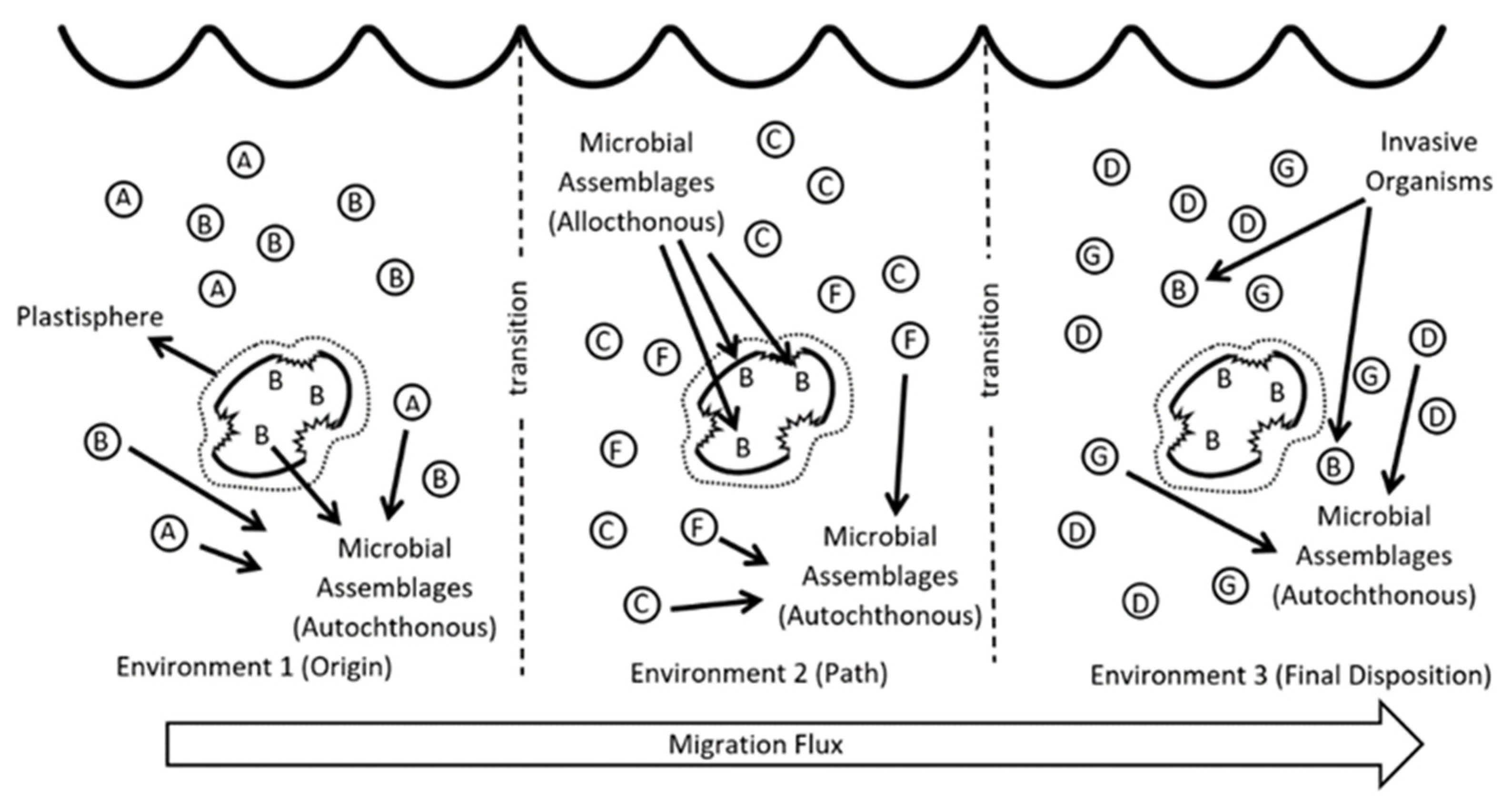
Figure 1. Transport of invasive species during microplastic migration along different sites (The varied strains of bacteria are represented by different letters: A, B, C, D, F and G).
Some bacterial groups, such as the phyla Bacteroidetes, Proteobacteria, Cyanobacteria and Firmicutes, are more often found colonizing MPs than other types of particles [25,33][1][17]. Certain bacterial taxa, then, seem to be more resistant to the toxic compounds of the plastic matrix, either naturally, or because of ready metabolic adaptation. The latter may be linked to processes such as attachment, degradation or chemotaxis [25,33][1][17].
Under the protective impact of the plastisphere, MPs can translocate the local microbiota to other areas, “rafting’’ microorganisms from their origins to other ecosystems [52,59][5][13]. Plastic items produced by humans and discharged into the marine environment as wastes can therefore be responsible for the migration and transportation of allochthonous species in aquatic environments (Figure 1). In this way, it has been suggested, pollution-resistant [64][20] or antibiotic-resistant [64,65][20][21] microbial groups may spread worldwide [52,66][5][22].
Human and non-human pathogenic bacteria have been detected in the plastisphere, again indicating the importance of this protective milieu for disease transmission. One of those most commonly reported is the genus Vibrio, which contains species pathogenic to humans [67][23] and to crustaceans [68][24]. E. coli pathotypes have also been detected in marine plastispheres [69][25]. In addition, micro-algae and cyanobacteria responsible for algal blooms have been implicated in plastisphere-associated transfer [33][17]. The adherent organisms may be released from the plastisphere when it breaks down because of a change in environmental conditions or through the action of biodegradative organisms within it.
2. The Plastisphere Micro-Niche and Biodegradation
According to Ward et al. (2022) [70][26], there are significant changes in colony formation during the first weeks of plastisphere production, revealing a complex ecological succession during the period of colonization of the micro-niche. Erni-Cassola et al. (2020) [71][27] reported that bacteria capable of using hydrocarbons as a carbon source play an important role in the initial stages of the process of colonization of the plastic surface. Similarly, Teughels et al. (2006) [72][28] and Rummel et al. (2017) [45][29] believe that the first stages of ecological succession and resulting colonization are dominated by species more adapted to more hostile environments, pioneer substrate-specific taxa capable of degrading plastics, later replaced by more generalist biofilm component species [41][30]. Initially, bacteria and diatoms are the major biofilm components, but other organisms, such as microalgae, fungi and heterotrophic protists (flagellates and ciliates), also populate these micro-niches. They may bring other degradative activities to the plastisphere. Degradation of plastics in the marine environment has, however, been less studied than in freshwater or soil, and degradation rates are practically unknown [73][31]. Goudriaan et al. (2023) [74][32] discuss the problems and deficiencies of studies on biodegradation of plastics in the marine environment. Unambiguous proof of microbial degradation and quantification of the normally low degradation rates are two problematic areas. There are, however, numerous studies of biodegradation in other environments [75,76,77,78,79,80,81,82][33][34][35][36][37][38][39][40].
During biofilm maturation and microbial succession, biological transformations occur in parallel with physical and chemical changes that include degradation and oxidation of the polymer itself by microbiota living on the plastic particle surface in an ecologically complex multilayer micro ecosystem [46][41]. Microorganisms may be both stimulated and inhibited within the highly variable physicochemical microclimate of the MP surface, depending on the additives and contaminants present. The plastic biodegradation process depends on many variables, such as polymer composition and resulting molecular weight, particle surface physical characteristics and environmental parameters [83,84,85][42][43][44]. The process has been evaluated by monitoring a varied group of parameters. These are substrate weight loss, changes in mechanical properties and/or chemical structure of the polymer, and the percentage of carbon dioxide released. The initial tests of microbiological biodegradation sought to prove that microbial activity would result in physical changes in the polymer matrix, such as mechanical strength, degree of crystallinity and water absorption [86,87][45][46]. The various plastic biodegradation processes are directly related to the compositional particularities of each polymer, just as the active sites of enzymes are particular to their specific substrate configurations. The main polymeric compounds can be divided into three groups: polymers whose basic molecule is formed by linear carbon chains (polyethylene—PE, polypropylene—PP, polystyrene—PS, and polyvinyl chloride—PVC); polymers with ester-linked backbones and side chains (polyethylene terephthalate—PET, and polyurethane—PU); and polymers with hetero/carbamate(urethane) linkages (polyurethanes—PUs) (Figure 2).
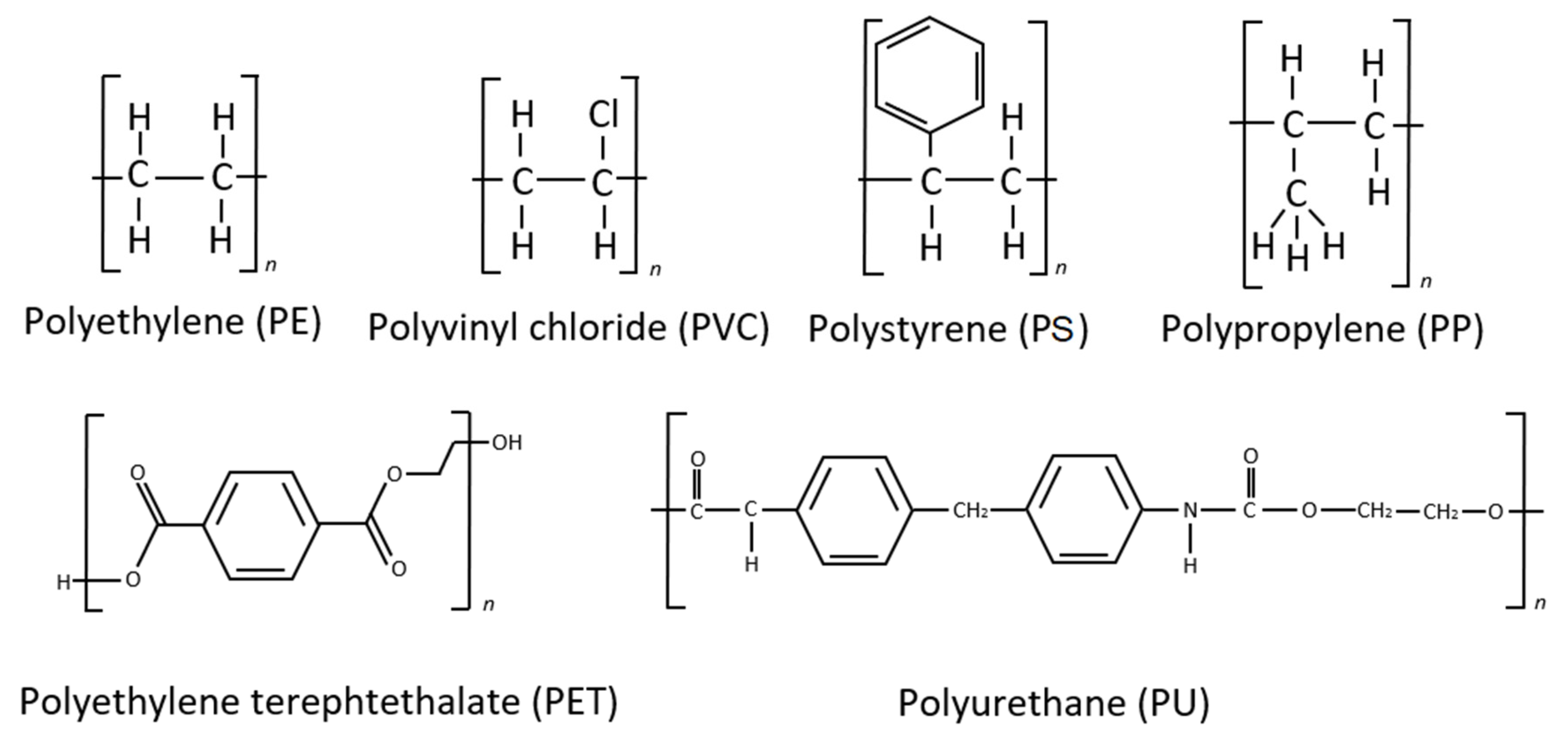
Figure 2.
Structures of major commercial synthetic polymers.
References
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146.
- Da Fonseca, E.M.; Gaylarde, C.C.; Baptista Neto, J.A.; Camacho Chab, J.C.; Ortega-Morales, O. Microbial interactions with particulate and floating pollutants in the oceans: A review. Micro 2022, 2, 257–276.
- Almeida, M.P.; Gaylarde, C.C.; Baptista Neto, J.A.; Neves, C.V.; Fonseca, E.M. Particulate and Floating Pollutants in the Oceans. Encycl. J. 2022, 1, 1.
- Amaral-Zettler, L.; Zettler, E.; Slikas, B.; Boyd, G.; Melvin, D.; Morrall, C.; Proskurowski, G.; Mincer, T. The biogeography of the Plastisphere: Implications for policy. Front. Ecol. Environ. 2015, 13, 541–546.
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the plastisphere. Nat. Rev. Microbiol. 2020, 18, 139–151.
- Wang, F.; Zhang, M.; Sha, W.; Wang, Y.; Hao, H.; Dou, Y.; Li, Y. Sorption behavior and mechanisms of organic contaminants to nano and microplastics. Molecules 2020, 25, 1827.
- Bhagwat, N.R.; Owens, S.N.; Ito, M.; Boinapalli, J.V.; Poa, P.; Ditzel, A.; Kopparapu, S.; Mahalawat, M.; Davies, O.R.; Collins, S.R.; et al. SUMO is a pervasive regulator of meiosis. Elife 2021, 10, 57720.
- Mughini-Gras, L.; van der Plaats, R.Q.; van der Wielen, P.W.; Bauerlein, P.S.; de Roda Husman, A.M. Riverine microplastic and microbial community compositions: A field study in the Netherlands. Water Res. 2021, 192, 116852.
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Let. 2015, 10, 124006.
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576.
- Dąbrowska, A. A roadmap for a Plastisphere. Mar. Poll. Bull. 2021, 167, 112322.
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A.; et al. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359.
- Li, S.; Wang, T.; Guo, J.; Dong, Y.; Wang, Z.; Gong, L.; Li, X. Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J. Hazard. Mater. 2021, 1, 415.
- Wang, R.; Neoh, K.G.; Shi, Z.; Kang, E.T.; Tambyah, P.A.; Chiong, E. Inhibition of escherichia coli and proteus mirabilis adhesion and biofilm formation on medical grade silicone surface. Biotechnol. Bioeng. 2011, 109, 336–345.
- Sharma, S.; Basu, S.; Shetti, N.; Nadagouda, M.; Aminabhavi, T. Microplastics in the environment: Occurrence, perils, and eradication. Chem. Engin. J. 2020, 408, 127317.
- Reisser, J.; Shaw, J.; Hallegraeff, G.; Proietti, M.; Barnes, D.K.; Thums, M.; Wilcox, C.; Hardesty, B.D.; Pattiaratchi, C. Millimeter-sized marine plastics: A new pelagic habitat for microorganisms and invertebrates. PLoS ONE 2014, 9, 100289.
- Dussud, C.; Meistertzheim, A.L.; Conan, P.; Pujo-Pay, M.; George, M.; Fabre, P.; Coudane, J.; Higgs, P.; Elineau, A.; Pedrotti, M.L.; et al. Evidence of niche partitioning among bacteria living on plastics, organic particles and surrounding seawaters. Environ. Poll. 2018, 236, 807–816.
- Oberbeckmann, S.; Löder, M.; Labrenz, M. Marine microplastic-associated biofilms—A review. Environ. Chem. 2015, 12, 551–562.
- Debroas, D.; Mone, A.; Ter Halle, A. Plastics in the North Atlantic garbage patch: A boat-microbe for hitchhikers and plastic degraders. Sci. Total Environ. 2017, 599, 1222–1232.
- Yang, Y.; Liu, G.; Song, W.; Ye, C.; Lin, H.; Li, Z.; Liu, W. Plastics in the marine environment are reservoirs for antibiotic and metal resistance genes. Environ. Intern. 2019, 123, 79–86.
- Zhang, S.; Liu, X.; Hao, X.; Wang, J.; Zhang, Y. Distribution of low-density microplastics in the mollisol farmlands of northeast China. Sci. Total Environ. 2020, 708, 135091.
- Bowley, J.; Baker-Austin, C.; Porter, A.; Hartnell, R.; Lewis, C. Oceanic hitchhikers–assessing pathogen risks from marine microplastic. Trends Microbiol. 2021, 29, 107–116.
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers. 2018, 4, 1–19.
- De Souza Valente, C.; Wan, A.H. Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J. Invertebrate Pathol. 2021, 181, 107527.
- Silva, M.M.; Maldonado, G.C.; Castro, R.O.; de Sá Felizardo, J.; Cardoso, R.P.; Dos Anjos, R.M.; de Araújo, F.V. Dispersal of potentially pathogenic bacteria by plastic debris in Guanabara Bay, RJ, Brazil. Mar. Poll. Bull. 2019, 141, 561–568.
- Ward, C.S.; Diana, Z.; Ke, K.M.; Orihuela, B.; Schultz, T.P.; Rittschof, D. Microbiome development of seawater-incubated pre-production plastic pellets reveals distinct and predictive community compositions. Front. Mar. Sci. 2022, 8, 2047.
- Erni-Cassola, G.; Wright, R.J.; Gibson, M.I.; Christie-Oleza, J.A. Early colonization of weathered polyethylene by distinct bacteria in marine coastal seawater. Microb. Ecol. 2020, 79, 517–526.
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81.
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267.
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Inter. 2020, 136, 105411.
- Zhu, X. The plastic cycle–an unknown branch of the carbon cycle. Front. Mar. Sci. 2021, 7, 1227.
- Goudriaan, M.; Morales, V.H.; van der Meer, M.T.; Mets, A.; Ndhlovu, R.T.; van Heerwaarden, J.; Simon, S.; Heuer, V.B.; Hinrichs, K.U.; Niemann, H. A stable isotope assay with 13C-labeled polyethylene to investigate plastic mineralization mediated by Rhodococcus ruber. Mar. Poll. Bull. 2023, 186, 114369.
- Kathiresan, K. Polythene and Plastics-degrading microbes from the mangrove soil. Rev. De Biol. Trop. 2003, 51, 629–633.
- Skariyachan, S.; Patil, A.A.; Shankar, A.; Manjunath, M.; Bachappanavar, N.; Kiran, S. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym. Degrad. Stab. 2018, 149, 52–68.
- Munir, E.; Harefa, R.S.M.; Priyani, N.; Suryanto, D. Plastic degrading fungi Trichoderma viride and Aspergillus nomius isolated from local landfill soil in Medan. IOP Conf. Ser. Earth Environ. Sci. 2018, 126, 012145.
- Puglisi, E.; Romaniello, F.; Galletti, S.; Boccaleri, E.; Frache, A.; Sandro, P. Selective bacterial colonization processes on polyethylene waste samples in an abandoned landfill site. Sci. Rep. 2019, 9, 14138.
- Bardají, D.K.R.; Furlan, J.P.R.; Stehling, E.G. Isolation of a polyethylene degrading Paenibacillus sp. from a landfill in Brazil. Arch. Microbiol. 2019, 201, 699–704.
- Cárdenas Espinosa, M.J.; Colina Blanco, A.; Schmidgall, T.; Atanasoff-Kardjalieff, A.K.; Kappelmeyer, U.; Tischler, D.; Pieper, D.H.; Heipieper, H.J.; Eberlein, C. Toward Biorecycling: Isolation of a Soil Bacterium That Grows on a Polyurethane Oligomer and Monomer. Front. Microbiol. 2020, 11, 404.
- Janatunaim, R.Z.; Fibriani, A. Construction and cloning of plastic-degrading recombinant enzymes (MHETase). Recent. Pat. Biotechnol. 2020, 14, 229–234.
- Roy, R.; Mukherjee, G.; Das Gupta, A.; Tribedi, P.; Sil, A.K. Isolation of a soil bacterium for remediation of polyurethane and low-density polyethylene: A promising tool towards sustainable cleanup of the environment. Biotech 2021, 11, 29.
- McGivney, E.; Cederholm, L.; Barth, A.; Hakkarainen, M.; Hamacher-Barth, E.; Ogonowski, M.; Gorokhova, E. Rapid physicochemical changes in microplastic induced by biofilm formation. Front. Bioeng. Biotechnol. 2020, 8, 205.
- Albertsson, A.C.; Anderson, S.O.; Karlsson, S. Mechanism of biodegradation of polyethylene. Polym. Degrad. Stab. 1987, 18, 73–87.
- Ammala, A.; Bateman, S.; Deana, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Colin, P.; Leong, K.H. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049.
- Harrison, J.P.; Boardman, C.; O’Callaghan, K.; Delort, A.M.; Song, J. Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review. R. Soc. Open Sci. 2018, 5, 171792.
- Pirt, S.J. Microbial degradation of synthetic polymers. J. Chem. Technol. Biotechnol. 1980, 30, 176–179.
- Albertsson, A.C.; Karlsson, S. Aspects of biodeterioration of inert and degradable polymers. Int. Biodeterior. Biodegrad 1993, 31, 161–170.
More
