Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Francisco Jose Alguacil and Version 2 by Conner Chen.
Hydrogen sulfide is a toxic and corrosive gas; thus, in order to mitigate its environmental impact, its capture and removal from various emitting sources, natural and anthropogenic, is of a necessity.
- absorption
- adsorption
- hydrogen sulfide
- membranes
1. Absorption Processes to Remove Hazardous H
2
S from Gas Streams
This technology uses both chemical and physical solvents and is one of the most preferred to remove H2S from these gas streams. The use of chemical solvents mitigates the presence of H2S in the corresponding phase. Among these chemicals, alkanolamine solutions know a wide usefulness because, together with toxic H2S, the solvent removes CO2 [1][6]. One step ahead the use of conventional solvents, ionic liquids are considered as their reliable alternatives. The well-known singularities or properties that these chemicals make are gaining positions on H2S removal [2][7]. The use of mixtures (AAILs) of tertiary amines and ionic liquids (amino acid) to remove H2S was investigated in [3][8]. Among these compounds, tetramethylammonium arginine and tetramethylammonium glycine, [N111][Arg] and [N1111][Gly], respectively, presented H2S removal rates of 100%. The rate of gas removal is increased when transferring protons between the ionic liquid and the tertiary amine. Larger-scale applications of the method seemed to be unpractical due to the costs and complex synthesis of the ionic liquids. In term of costs, deep eutectic solvents (DESs) are an option to ionic liquids. Interaction between H2S and DES (basic) does not imply chemical reaction. Functionalizing these deep eutectic solvents with some chemicals, including amines, and oxidizing reagents produced an increase in the absorptive and regenerative properties of the compounds [4][9]. The addition of polyethyleneimine to deep eutectic solvents was investigated [5][10], the mixture having 90% H2S removal efficiencies after four consecutive absorption–regeneration cycles. Nanofluids also emerged as potential absorbents for H2S. These absorbents are formed by dispersion of several inorganic compounds (SiO2, Al2O3) in the form of nanoparticles or graphene oxide and carbon nanotubes into organic solvents (monoethanolamine, diethanolamine, etc.) [6][11]. Removal of H2S via the use of Fe-Monoethanolamine-BmimCl solution with inert nanoparticles (SiO2) presented good perspectives, though H2S removal efficiency decreased after continuous use (cycles) [7][12]. Some of the difficulties presented by conventional solvents appeared to be resolved with the use of these ionic liquids and deep eutectic solvents; however, some of the properties of these compounds, i.e., high viscosity, can be considered a drawback since it reduces mass transfer coefficients and thus causes an increase in energy demand [8][18]. The use of mixtures of these compounds can improve the removal of the toxic gas; however, the greatest negative impact of these mixtures is the lack of maintaining the removal properties after continuous use [9][19]. A new perspective has been raised in terms of improving the regeneration step to avoid this being lost in the absorption properties. Various triethylenetetramine functionalized ionic liquids (TETAH-ILs) are mixed with ethylene glycol (EG) to investigate their performance on H2S absorption [10][20]. The results showed that the mixture 10% [TETAH][BF4]-EG presented the best absorption capacities (1.128 mol H2S/mol IL) at 30 °C and 100 mL/min. Removal of H2S is attributed to the formation of H2S-IL compounds via hydrogen bonds. A bubble absorption column is used to investigate hydrodynamics of CO2 and H2S removal from pure water and water containing nanofluids dispersed with neat and OH- and NH2-functionalized multiwalled carbon nanotubes [11][21]. Sodium dodecyl sulfate is used as surfactant and stabilizer. Maximum CO2 removal and H2S removal are found to be 0.0038 mmol/m2·s and 0.056 mmol/m2·s using NH2-MWCNTs/nanofluid, respectively. The improvement in the absorption properties of diethanolamine with respect to the removal of H2S from sour industrial off gas was investigated in [12][22]. The influence of lean amine H2S impurity (LAHI), lean amine temperature (LAT), and column pressure (CP) on H2S removal was studied. The increase of LAHI and LAT is detrimental for H2S removal from the gas stream and LAT, LAHI (83%) being the key factor on H2S removal, whereas LAT (15%) and CP (2%) have a minor impact on this efficiency. The next investigation used machine-learning operations to study the solubility of H2S in fifteen ionic liquids [13][23]. No less than six machine-learning operations were used, and the respective results were compared. The conclusions showed that the least-squares support vector machine predicted H2S solubility into the ionic liquids well with R2 (0.99798), RMSE (0.01079), MSE (0.00012), RRSE (6.35%), RAE (4.35%), MAE (0.0060), and AARD (4.03). It was found that H2S solubility decreased with temperature and has a direct dependence with the pressure. Ionic liquids such as [OMIM][Tf2N] are the best choice for H2S capture. The next investigation [14][24] explored various aspects of the processing and technologies used in acid gas removal (AGR). The work summarized processing by chemical absorption and mechanisms involved in the removal process; it also showed the main amine-based solvents currently used in such tasks. Absorption by physical methods is also discussed, summarizing pros and cons of the most used absorbents. Industrial applications of AGR processes were considered. The removal of H2S from industrial gas streams using an iron/copper bimetallic catalytic oxidation desulfurization system was investigated in [15][25]. The absorbent is formed by adding N-methyl pyrrolidone (NMP) and CuCl2 aqueous solution to an iron-based ionic liquid (Fe-IL). The acidity and viscosity of the system are greatly reduced by addition of NMP and water, improving gas–liquid mass transfer efficiency. The presence of the copper(II) salt increased the oxidative properties of the solution, allowing for the improvement of the Fe3+ catalytic influence on the oxidation of H2S to the monomer form of sulfur.2. Adsorption Processes to Remove Hazardous H
2
S from Gas Streams
Various materials: metal oxides, zeolites, activated carbon, metal organic frameworks (MOFs), biochar, mesoporous silica, ash, and composite materials have been investigated in the removal of H2S from gas streams. Selection of these materials is based in some of their properties, such as elevated uptake capacity, selectivity, and thermal and mechanical stability with respect to the removal of this toxic gas [16][26]. A mixture of biosolid (sewage) and surfactant (pluronic surfactant F127, heated at 950 °C) was used for the adsorption of H2S in [17][27]. It was shown that the presence of the surfactant increased the mesopore volume and carbon content of the biosolid, whereas the treatment at 950 °C developed the micropores, leading to the dispersal of the catalytic sites (Ca and Fe oxides) responsible for the gas adsorption, and increased the nitrogen atoms of pyrrolitic nature. Besides adsorption, the catalytic sites provide an environment to promote the oxidation of H2S to elemental sulfur. As a consequence, the breakthrough capacity is greatly improved (250% better than that presented by the biosolid alone); moreover, this capacity (221.2 mg/g) is better than those derived from other adsorbents such as STIX® (North Tonawanda, NY, USA) (201 mg/g), S-208 (36 mg/g), or Centaur® (Mumbai, India) (176 mg/g). Metal oxides presented have good properties for adsorbing H2S; however, there is a continuous effort to improve their characteristics in this important field. Adsorption of H2S by molybdenum(IV) oxide nanoparticles is described in the literature [18][28]. The best results are achieved at 0.081 and 0.074 g H2S/g, at a temperature of 85 °C, pressure of 16 bar, and superficial velocity of 0.018 m/s. The above capacities are obtained when the adsorbent presents non-spherical and spherical shapes and using an initial H2S concentration of 43 ppm in the feed gas stream. MnO2 is the most effective adsorbent compared with other composite nano-sized metal oxides such as NiO/TiO2, CoO/TiO2, graphite oxide/ZnO, and CuO/TiO2. Zeolites are other group of adsorbent materials of a wide use in several fields; some of the properties of these materials, i.e., elevated porosity and surface areas and porosities, are responsible for the wide use of these materials, including the removal of H2S. Y and ZSM-5 zeolites’ properties (including surface and pore structure) have been modified by the use of magnetite nanoparticles [19][29], being the performance, with respect to the H2S removal, of both adsorbents at the temperatures in the 100–300 °C range at a pilot scale. Activated carbons and biochars are other types of adsorbents with active adsorption sites. Jute thread waste (a cellulosic biomass) activated with KOH served to yield a N–S-rich nanoporous carbon [20][34]. The carbon has a pore volume of up to 1.50 cm3/g and a surface area of up to 2580 m2/g. Within this material, increasing the pressure increased the H2S adsorption capacity by 1 bar (19.1 mmol/g), 10 bar (32.6 mmol/g), and 35 bar (45.0 mmol/g). Again, the presence of pyrrolic-N atoms in the material helps with H2S uptake onto it, this being attributable to hydrogen bonds; at the same time, negatively charged sulfur atoms around the pyrrolic-N atoms are responsible for a physisorption process. This material presented an 84% of adsorption regeneration after five cycles. Using a peanut shell as precursor, impregnated with copper and activated by KOH, an activated carbon was formed and used to investigate the removal of H2S [20][34]. The best sample of the activated carbon has 1523.2 m2/g of surface area, with 97.6 mg/g of gas uptake. The non-linear Langmuir isotherm model best fits the experimental results:3. Membranes and Membrane Contactors to Remove Hazardous H
2
S from Gas Streams
The membranes as a separation technology are widely used in a variety of industries, and this wide usefulness is attributable to the properties or characteristics of the technology: modular aspects, easiness of operation, low environmental impact, etc., that in many aspects surpassed the offer presented by other separation technologies, this being specially noted when the operation, i.e., H2S removal, is accomplished in areas or locations where communications are not easy [31][47]. There are some recent publications reviewing the use of these membrane technologies on the treatment of gases [32][33][34][35][48,49,50,51], but surprisingly, they are dedicated to industrial gases instead of H2S; some of the gases mentioned in these reviews are: nitrogen, hydrogen, oxygen, CO2, etc. In the case of H2S, polymeric membranes are the candidates to resolve this important industrial issue. The permeation of gas across these types of membranes is ruled by diffusion, and a series of investigations [36][37][52,53] have mentioned that plasticization of the membrane performs well in the removal of H2S when the gas in the stream is present at high concentrations; moreover, this plasticization improves the selective separation of H2S over methane. This selective separation is attributed to the transport mechanism, which in the case of H2S is of sorptive in nature, and this separation is negatively influenced by diffusion. The transport ruled by solubility is particularly important in the case of rubbery polymers, and this is a key rule in the separation of H2S from methane. It is reported [38][54] that the variation of the cross-linking density tuned the usefulness of polyethylene glycol-based membranes. This change in the membrane properties significantly improves the selectivity in the pairs H2S/methane and CO2/methane. Computational methods have developed a series of tools to improve the knowledge and optimize the use of these membrane technologies in the capture of gases [39][57]. Another implication of membrane technologies on the removal of gases is their use to act as an interface between the different streams, liquid and gases, feeding the removal process. These membranes, preferably with high porosity, increased the gas flow through the membrane structure. In practice, hollow fiber modules are the most used devices; under operation, the gas flowing in the shell side diffuses across the membrane’s pores to the tube side; here, the gas is conveniently absorbed by a solution, whereas these modules can be operated in co-current and counter-current form, and the latter is usually preferred since it gives a better contact between both operating phases. This counter-current configuration is shown in Figure 1.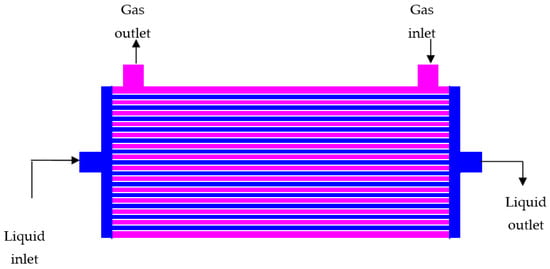
Figure 1.
Hollow fiber membrane module for gas removal in counter-current operational mode.
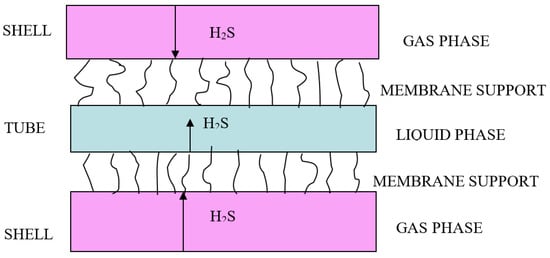
Figure 2.
Mass transfer in the hollow fiber module. Multiple curve black lines represented membrane micropores.
