Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Jelena Ćirić.
American foulbrood (AFB) is exclusively an infectious disease of honey bee larvae (Apis mellifera) and their subspecies that is spread easily and rapidly and is often present in apiaries. Due to the resistance and pathogenicity of the bacterial causative agent of the disease, which has considerable epizootiological and economic significance for beekeeping, AFB was classified as a highly dangerous, infectious animal disease by the World Organization for Animal Health (WOAH). Considering the severity of the infection, a frequent occurrence, rapid and easy spread, epizooty and enzooty are common.
- American foulbrood
- etiology
- diagnosis
- control
- Apis mellifera
1. Etiology of the Causative Agent of the Disease
The vegetative form of the causative agent of AFB is the Gram-positive, mostly mobile, rod-shaped bacterium, genus Paenibacillus, species Paenibacillus larvae (P. larvae), formerly known as Bacillus larvae White [10][1]. In microscopic preparations of newly dead larvae, the bacilli are mostly individual, while in cultures, depending on the phase of growth on artificial nutrient media, they are present in the form of short or long chains. Endospores of P. larvae appear the most in older pathological processes, larvae degradation (characterized by an amorphous, glutinous, dilatable mass that dries to a scale) [11,22,23][2][3][4] and in older cultures on artificial culture medium (Figure 1). There can be over one billion endospores in one cell of a diseased brood comb and in a single dead larva [2][5]. The endospores are elliptical, mostly central, highly resistant to extreme temperatures and other physicochemical agents, and they are the only infectious cause of AFB [4][6]. In normal conditions and in pathological material, the remains of dried larvae, scales, old comb in the bee hive, and endospores remain infectious for up to thirty-five years, and endospores released from wax, propolis, honey, and other honey bee products remain so for over seventy years [24][7].
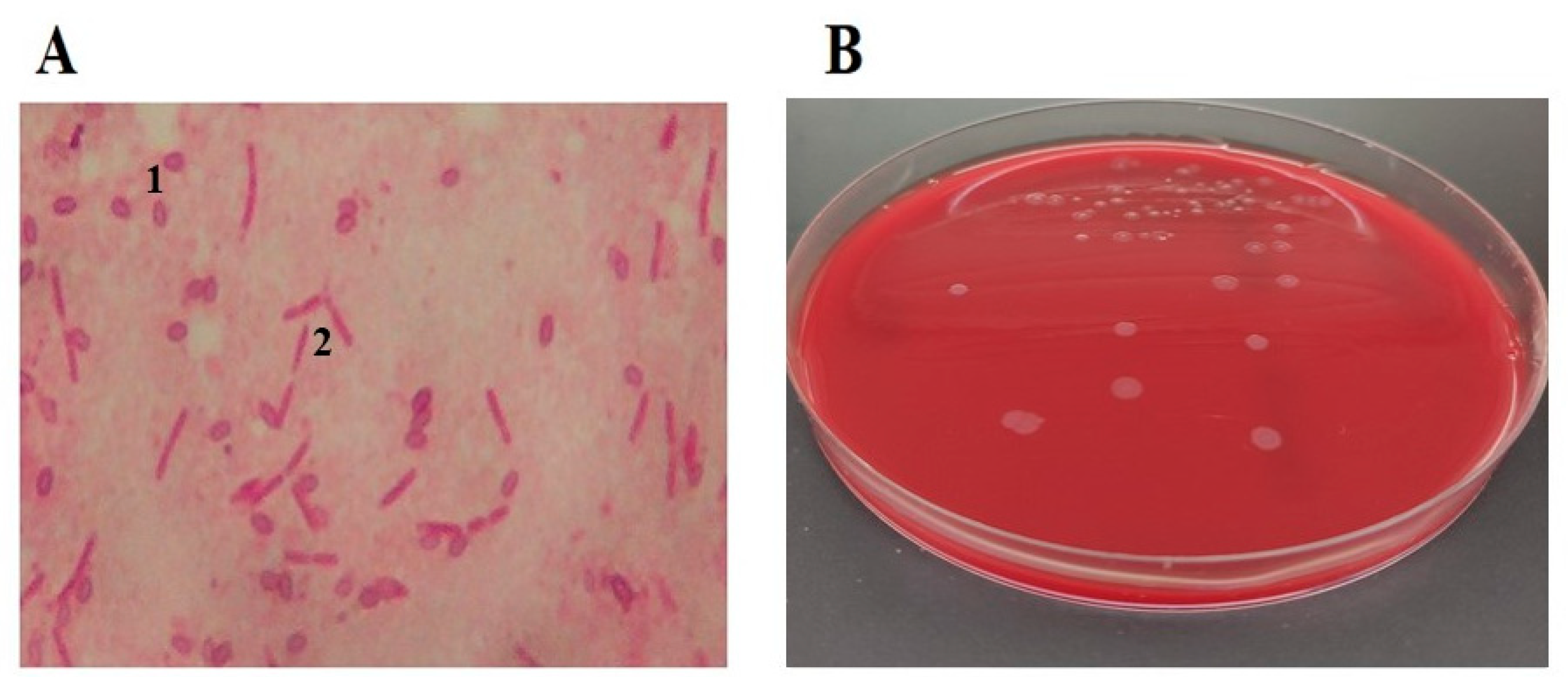
Figure 1.
Microscopic view (1000×) of
P. larvae
endospores 1 and vegetative cells 2 (
A
) and
P. larvae
cultured on Columbia Blood Agar (
B
).
By applying molecular techniques, several genotypes of P. larvae can be confirmed [17,22][3][8]. The genotypes ERIC I and ERIC II of P. larvae have been the most commonly isolated genotypes in the world [2,17,25][5][8][9]. P. larvae genotypes ERIC III and ERIC IV have not been isolated for a long time, but there are several isolates in bacterial culture collections [2,22][3][5]. According to Beims et al. [26][10], a new ERIC genotype has been recently discovered—P. larvae ERIC V—in samples of Spanish honey (Table 1). All five genotypes have different forms of endospores. Morphologically, the endospores of genotypes ERIC I and II have a smooth surface, while the endospores of genotype ERIC III–V have longitudinal ridges [26][10]. All of those are pathogenic for bee larvae, give the same clinical picture (amorphous, glutinous, dilatable mass that dries into a scale), but are different because of the bacterial colony appearance, pigmentation, hemolysis, fermentation of carbohydrates, and virulence [2,4,22,25,26,27,28][3][5][6][9][10][11][12].
Table 1.
P. larvae
genotypes and their characteristics.
| Genotype | ERIC I | ERIC II | ERIC III | ERIC IV | ERIC V |
|---|---|---|---|---|---|
| Virulence | Kill larvae within 12 days | Kills larvae within 7 days | Kills larvae within 7 days | Kills even after 3 days | |
| Frequency | Most frequent genotype, found throughout the world | Isolated worldwide, especially in Europe | Not identified in recent decades | Identified in Spain | |
| References | [2,22,29][3][5][13] | [2,22,29][3][5][13] | [2,[22,329]][5][13] | [26][10] | |
It has been shown that different genotypes of
P. larvae
have different virulence.
2. Control of the Disease
Somerville et al. [45][14] recommend good agricultural practice (GAP) and good hygienic practice (GHP) in beekeeping. These are prevention measures that include all general and preventive procedures in order to prevent the disease from getting into non-infected apiaries. In case of clinical signs or suspicion of AFB, it is the duty and legal obligation of everyone in the chain of biological and food production, from beekeepers to veterinarians in the field, veterinarians in the laboratory, and veterinary inspectors, to take appropriate measures and procedures. Good practices include: apiary hygiene; regular checks of the colonies; comb replacement; current disinfection while working in apiaries; inspection of purchased bee colonies and foraging colonies; using sterilized comb foundations produced according to hazard analysis and critical control points (HACCP) systems; laboratory inspection of supplemental feed; and special attention when accepting feral swarms [7,13,43,44,46][15][16][17][18][19]. The first and most important step in the suppression of AFB is early disease detection by beekeepers [15,46,47][19][20][21]. A thorough inspection is necessary in the spring if the disease has not been previously diagnosed. When symptoms of the disease appear, during the inspection of each colony, all beekeeping tools and equipment (beekeeping knife, gloves, blouse, etc.) that are used must be disinfected or during the inspection researchers must use disposable equipment that can be safely destroyed after the inspection (gloves). If the disease has occurred nearby, examination should be conducted more often and with the utmost caution. If the disease is detected in one colony in an apiary, all the colonies in that apiary must be examined, as well as those in apiaries that are located within a 3 km radius [6][22]. If AFB is suspected, the veterinary inspector must be informed immediately in order that all actions and measures needed to suppress and eradicate the disease are undertaken in a fast and efficient way [6,7,11][2][15][22]. AFB cannot be cured and so in accordance with legal procedures, in case of disease and by order of persons authorized by law, radical measures must often be taken. Namely, operators must close and inspect the infected apiary; close the hive entrance and suffocate the bees in the infected bee colony when all the bees have entered the hive (usually in the evening); burn the frames with a comb, as well as the honey and bees from the infected colony; bury all the mentioned materials and dead bees, and conduct thorough disinfection [8,10,43][1][17][23] or, less often, deploy the shaking bee hives measure [7][15]. After thorough mechanical cleaning, usable (but still new, preserved) hives are burnt with a blowlamp and disinfected with a 2–6% NaOH solution (caustic soda), KOH, Na2CO3, or alkaline hypochlorite solution (5% NaClO in the above solutions), heated to 80 °C. After applying one of the aforementioned solutions, the hives are immersed for 5–15 min (this increases exposure to the equivalent of two hours of spraying), then washed with warm water and dried [43,44][17][18]. A variety of different studies [43,44][17][18] after mechanical cleaning suggested the need to disinfect tools and other equipment with a Na2CO3 solution (1 part Na2CO3 and 5 parts water) or a 0.5% hypochlorite solution (NaClO) for twenty minutes. Additionally, they suggest to disinfect the apiary area with a solution of 10–20% Ca(OH)2. Over the next two months, the remaining bee colonies must be monitored closely while enhancing the disinfection of all the tools used to inspect every hive. If there are no new clinically suspicious AFB colonies during this time or if the suspicious colonies are not confirmed as diseased after laboratory examination, there is considered is considered to be no infection [6][22]. One method still in use for the eradication of AFB in some countries is the shaking method [7][15]. Considering that AFB is a disease of bee brood, not adult bees, it means shaking/transferring adult bees from the infected community to new disinfected hives and bee combs free from P. larvae endospores in the rest of the beehives in quarantine. Everything that is infected in hives (wax, honey, pollen, propolis, litter) is destroyed. Special/other equipment is always used in the implementation of these measures [7][15]. AFB is first suspected based on clinical signs of the disease in the apiary, but the diagnosis of AFB is based on laboratory identification of the pathogen [2][5]. During preventive examinations (monitoring) for the presence of AFB causative agents in the laboratory, in the case of a positive diagnosis, the colonies and apiaries must be clinically examined and legal measures taken [4,7,8,44,47][6][15][18][21][23]. While dealing with material suspected of harboring AFB, all biosecurity measures, risk analyses and assessments must be applied to reach the standard for biorisk management in veterinary laboratories and animal facilities [8][23].3. Differential Diagnostics of the Disease
At first glance, clinical symptoms AFB can be mistaken for sacbrood disease, European foulbrood, and varroosis, mostly in the early spring [8,12,30,46,52][19][23][24][25][26]. Sacbrood disease is a viral disease of the bee brood, mostly benign, in which larvae do not pupate. Unlike AFB (in which dead larvae transformed into an amorphous, gelatinous and extensible mass and, at the end of the process, often in the spring, into a scale), in the case of sacbrood disease, dead grey-brown larvae under the cap are easily pulled out of the cell and take the shape of a bag (sac), Figure 3B. If the sacbrood process is older, due to drying, the larva turns into a scale, and the head and the last part of the body bend, and thus, it forms into a boat shape. In the early spring, with varroosis, under the cap of the cells of the bee brood, one can see one of the deaths of the often deformed developmental forms of the bee (pupa, young bee) and the presence of Varroa destructor that is most often united with viruses [12,46][19][24]. European foulbrood is both an uncapped and sealed brood disease. As such, so diseased larvae die in both uncapped and sealed broods. The causative agent of the disease is a bacterium of the genus Melissococcus, species Melissococcus plutonius (M. plutonius). Diseased larvae are yellowish, often bloated, limp, mushy or liquid; when pulled out of the cell, their chitin layer tears. Sometimes, larvae stop being white due to drying, they lose their pearly glow and segmentation, they do not transform into pupae, and bees can easily eject them from the hive [8,12,30,46][19][23][24][25]. In early spring, dead bee colonies can often be found with a sealed, unhatched brood, and plenty of food in the hive. Sometimes a doubt can be raised about the cause of the death of the colony, i.e., AFB and/or varroosis [12,46][19][24]. In the case of varroosis, after opening the caps, there is usually a formed pupa or young bee in the cell with one or more varoa mites. When it comes to AFB, there are no completed stages of bee development (pupae, young bee), but it is a late stage of the disease (the mass is dried out), and the cell is apparently empty. However, if we examine it carefully, we can find black and brown scales at the bottom, which is quite common for AFB disease processes older than two months [46][19]. WOAH has presented a broad outline of various diagnostic methods. However, due to differences in sensitivity, the most appropriate of the described methods should be selected [8][23]. In addition to classical microbiological and antibody-based techniques, molecular and physicochemical methods are used. A wide range of samples can be delivered to the laboratory (diseased and dead larvae, cell swabs, honey, pollen, royal jelly, wax, dead bees, food, and debris from the hive floor) [8,9,46][19][23][27]. The specific methodology applied depends on the type and nature of the materials delivered to the laboratory and the purpose of the examination (preventive examination, suspected disease, already confirmed presence of the disease) [9,16,25,46][9][19][27][28]. Within classical microbiological methods, microscopic determination of endospores of P. larvae and microbiological cultivation and isolation on nutrient media, with biochemical identification of the causative agent of AFB, are the most commonly applied methods [8,22,30][3][23][25]. There are several enriched selective media for P. larvae culture: P. larvae agar (PLA), Mueller–Hinton, yeast extract, glucose, KH2PO4, sodium pyruvate agar (MYPGP), brain–heart infusion medium supplemented with thiamine agar (BHIT), yeast extract agar (J-agar), and Columbia agar from sheep’s blood agar (CSA) [17][8]. Within the antibody-based techniques, the immunodiffusion test, fluorescent antibody technique (TFA), and enzyme-linked immunosorbent assay (ELISA) are applied [53,54,55,56][29][30][31][32]. In the identification of the causative agent of AFB, molecular techniques are applied. Polymerase chain reaction (PCR), real-time polymerase chain reaction (real-time PCR), and pulsed-field gel electrophoresis (PFGE) are the most commonly used in laboratories [2,4,16,22,25,30,46,47,49,57,58][3][5][6][9][19][21][25][28][33][34][35]. Real-time PCR analysis of the 16S rDNA gene of P. larvae represents an alternative, rapid diagnostic tool. As a part of scientific research work as well as genetic and epidemiological studies, methods of partial genome sequencing are more often applied in laboratory diagnostics. These are Multilocus sequence typing (MLST), multiple-locus variable-number tandem repeat analysis (MLVA), and high-throughput sequencing (HTS) [57][34]. It is also possible to detect P. larvae using microbiome analysis [59][36]. A method that can also be used to identify this pathogen is based on a physicochemical technique, namely the matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) [60][37]. The microscopic method of detecting P. larvae endospores is definitely less sensitive than microbiological isolation, but both methods are less sensitive than PCR methods [2,47,48,61,62][5][21][38][39][40]. The presence of P. larvae can be determined by microbiological isolation and PCR methods from bee colonies in which the disease has not occurred [16,25,46,47,49][9][19][21][28][33]. The existence of the endolysin cell-binding domain (CBD), which binds P. larvae [63][41], may lead to new methods for the identification of bacterial strains that cause AFB. Yones et al. [64][42] investigated the possibility of applying of hyperspectral technology as a new trend for immediate detection of AFB disease in honey bee larvae. Early detection of AFB disease in honey bee colonies is certainly of substantial importance as interchanging of colony components can spread easily AFB to healthy colonies.4. Prevention of the Disease
Legally prescribed measures must be implemented for the detection, monitoring, suppression, and eradication of AFB. Those are prescribed at national, regional and international levels [6,65][22][43]. Among other things, these include reporting diseases to competent authorities, conducting epidemiological studies, and monitoring the prevalence of diseases [6,7,8,11,65][2][15][22][23][43]. It is also a duty of all responsible people in the beekeeping production to regularly monitoring the health condition of bee colonies, to conduct inspections, to launch anti-AFB initiatives, to implement necessary legal measures, and to share any suspicions of AFB with the competent veterinary services [6,8,15,44,49,65][18][20][22][23][33][43]. When working in the apiary and with bee colonies, special attention should be paid to bee and colony health. It is necessary to conduct a detailed, comprehensive, and expert inspection of bees and bee colonies for diseases, with special attention paid to the presence of bee brood diseases, including AFB. This should be performed at least twice a year, in autumn, including sampling and culturing the bacteria (prior to wintering) and in spring (before bees begin to forage) [7,16][15][28]. The health status of apiaries or bee colonies should be observed before commencing any work. Every observed change in the brood, comb, or apiary, the beekeeper must take it seriously and, in case of occurrence, report it to the competent veterinary service. In that case, fast and reliable laboratory tests/diagnostics will be provided, which is a prerequisite for taking legal measures to prevent the spread, control, and eradication of the disease. Some suspected diseases of bees and bee broods, especially AFB, can only be confirmed by laboratory analysis. Therefore, it is obligatory to regularly perform diagnostic testing of the materials originating from any apiary that suspects AFB [30,64,66,67][25][42][44][45]. In the case of the AFB outbreak, all provisions that refer to measures and procedures in the early detection, monitoring, prevention, suppression, and eradication of the disease must be comply [4,7,8,44,65,68][6][15][18][23][43][46]. Bee production should be organized into associations, because this is the best way fight against the negligence of individual beekeepers. All participants in the process of bee production (beekeepers, veterinarians, fruit growers, agronomists, ecologists) must be educated through meetings, sharing experiences, and expert lectures. Individual beekeepers, beekeeping organizations, clinical veterinary services, laboratories, and veterinary inspectors must all work together to achieve this goal [68][46]. Figure 42 shows the destruction of infected bee colonies by burning and burying them to render them in order to implement a part of the measures aimed at eradicating the diseases. The disinfection of apiaries has also been shown to be another measure in the chain of disease eradication.
Figure 42. Burning and burying infected bee colonies after destroying the bees to control AFB (A) and disinfection of the apiary with a 10% Ca(OH)2 solution, in the evening hours, after stamping out AFB-infected bee colonies (B).
References
- White, G.F. The Bacteria of the Apiary: With Special Reference to Bee Diseases; Technical Series; USDA, Bureau of Entomology: Washington, DC, USA, 1906; Volume 14, pp. 1–50.
- Genersch, E. Paenibacillus larvae and American Foulbrood—Long since known and still surprising. J. Consum. Prot. Food Saf. 2008, 3, 429–434.
- Genersch, E.; Forsgren, E.; Pentikäinen, J.; Ashiralieva, A.; Rauch, S.; Kilwinski, J.; Fries, I. Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int. J. Syst. Evol. Microbiol. 2006, 56, 501–511.
- Yue, D.; Nordhoff, M.; Wieler, L.H.; Genersch, E. Fluorescence in situ hybridization (FISH) analysis of the interactions between honeybee larvae and Paenibacillus larvae, the causative agent of American foulbrood of honeybees (Apis mellifera). Environ. Microbiol. 2008, 10, 1612–1620.
- Genersch, E. American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol. 2010, 103, S10–S19.
- Ebeling, J.; Knispel, H.; Hertlein, G.; Fünfhaus, A.; Genersch, E. Biology of Paenibacillus larvae, a deadly pathogen of honey bee larvae. Appl. Microbiol. Biotechnol. 2016, 100, 7387–7395.
- Hasemann, L. How long can spores of American Foulbrood live? Am. Bee J. 1961, 101, 298–299.
- Morrissey, B.J.; Helgason, T.; Poppinga, L.; Fünfhaus, A.; Genersch, E.; Budge, G.E. Biogeography of Paenibacillus larvae, the causative agent of American foulbrood, using a new multilocus sequence typing scheme. Environ. Microbiol. 2015, 17, 1414–1424.
- Hristov, Y.V.; Le Roux, J.J.; Allsopp, M.H.; Wossler, T.C. Identity and distribution of American foulbrood (Paenibacillus larvae) in South Africa. J. Apic. Res. 2021, 1–8.
- Beims, H.; Bunk, B.; Erler, S.; Mohr, K.I.; Spröer, C.; Pradella, S.; Günther, G.; Rohde, M.; von der Ohe, W.; Steinert, M. Discovery of Paenibacillus larvae ERIC V: Phenotypic and genomic comparison to genotypes ERIC I-IV reveal different inventories of virulence factors which correlate with epidemiological prevalences of American Foulbrood. Int. J. Med. Microbiol. 2020, 310, 151394.
- Jończyk-Matysiak, E.; Popiela, E.; Owczarek, B.; Hodyra-Stefaniak, K.; Świtała-Jeleń, K.; Łodej, N.; Kula, D.; Neuberg, J.; Migdał, P.; Bagińska, N.; et al. Phages in Therapy and Prophylaxis of American Foulbrood—Recent Implications from Practical Applications. Front. Microbiol. 2020, 11, 1913.
- Neuendorf, S.; Hedtke, K.; Tangen, G.; Genersch, E. Biochemical characterization of different genotypes of Paenibacillus larvae subsp. larvae, a honey bee bacterial pathogen. Microbiology 2004, 150, 2381–2390.
- Rauch, S.; Ashiralieva, A.; Hedtke, K.; Genersch, E. Negative Correlation between Individual-Insect-Level Virulence and Colony-Level Virulence of Paenibacillus larvae, the Etiological Agent of American Foulbrood of Honeybees. Appl. Environ. Microbiol. 2009, 75, 3344–3347.
- Somerville, D.; Annand, N.; New South, W. Healthy Bees: Managing Pests, Diseases and Other Disorders of the Honey Bee. (Sydney, NSW?): Tocal College, NSW DPI (AgGuide: A Practical Handbook). 2016. Available online: http://ezproxy.nb.rs:2059/login.aspx?direct=true&db=e000xww&AN=1580990&site=eds-live (accessed on 3 January 2023).
- Locke, B.; Low, M.; Forsgren, E. An integrated management strategy to prevent outbreaks and eliminate infection pressure of American foulbrood disease in a commercial beekeeping operation. Prev. Vet. Med. 2019, 167, 48–52.
- Lindström, A.; Korpela, S.; Fries, I. The distribution of Paenibacillus larvae spores in adult bees and honey and larval mortality, following the addition of American foulbrood diseased brood or spore-contaminated honey in honey bee (Apis mellifera) colonies. J. Invertebr. Pathol. 2008, 99, 82–86.
- Formato, G.; Smulders, F.J. Risk management in primary apicultural production. Part 1: Bee health and disease prevention and associated best practices. Veter-Q. 2011, 31, 29–47.
- Rivera Gomis, J.; Bubnic, J.; Ribarits, A.; Moosbeckhofer, R.; Alber, O.; Kozmus, P.; Formato, G.; Muz, M.N. Good farming practices in apiculture. Rev. Sci. Tech. L’oie 2019, 38, 879–890.
- Matović, K.; Žarković, A.; Vidanović, D.; Debeljak, Z.; Vasković, N.; Šekler, M. American foulbrood disease in the southwestern part of Serbia. In Improving Beekeeping in Serbia; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 2016; pp. 105–119. (In Serbian)
- Tomljanović, Z.; Cvitković, D.; Pašić, S.; Volarević, B.; Tlak Gajger, I. Production, practices and attitudes of beekeepers in Croatia. Vet. Arh. 2020, 90, 413–427.
- Forsgren, E.; Laugen, A.T. Prognostic value of using bee and hive debris samples for the detection of American foulbrood disease in honey bee colonies. Apidologie 2014, 45, 10–20.
- Council Directive, 92/65/EEC, Annex A. Off. J. Eur. Union 1992, 268, 58–63.
- OIE. Chapter 3.2.2: American Foulbrood of Honey Bees (Infection of Honey Bees with Paenibacillus larvae. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; OIE: Paris, France, 2018; pp. 1–17.
- Lolin, M. Bolesti Pčela; Naučna knjiga: Beograd, Serbia, 1985; p. 12. (In Serbian)
- De Graaf, D.C.; Alippi, A.M.; Brown, M.; Evans, J.D.; Feldlaufer, M.; Gregorc, A.; Hornitzky, M.; Pernal, S.F.; Schuch, D.M.T.; Titĕra, D.; et al. Under the microscope. Diagnosis of american foulbrood disease in honeybees: A synthesis and proposed analytical protocols. Lett. Appl. Microbiol. 2006, 43, 583–590.
- Food and Agriculture Organization (FAO) of the United Nations, Roma. Good Beekeeping Practices: Practical Manual on How to Identify and Control the Main Diseases of the Honeybee (Apis Mellifera); Food and Agriculture Organization (FAO): Roma, Italy, 2020.
- Teixeira, É.W.; Guimarães-Cestaro, L.; Alves, M.L.T.M.F.; Message, D.; Martins, M.F.; da Luz, C.F.P.; Serrão, J.E. Spores of Paenibacillus larvae, Ascosphaera apis, Nosema ceranae and Nosema apis in bee products supervised by the Brazilian Federal Inspection Service. Rev. Bras. Èntomol. 2018, 62, 188–194.
- Noureddine, A.; Nizar, H. Prévalence et répartition de la bactérie Paenibacillus larvae (Agent causal de la Loque américaine) au niveau de quelques ruchers de la région centre d’Algérie. Nat. Technol. 2021, 25, 85–93.
- Alippi, A.M. A comparison of laboratory techniques for the detection of significant bacteria of the honey bee, Apis mellifera, in Argentina. J. Apic. Res. 1991, 30, 75–80.
- Peng, Y.-S.; Peng, K.-Y. A study on the possible utilization of immunodiffusion and immunofluorescence techniques as the diagnostic methods for American foulbrood of honeybees (Apis mellifera). J. Invertebr. Pathol. 1979, 33, 284–289.
- Otte, E. Contribution to the laboratory diagnosis of American foulbrood (AFB) of the honey bee with particular reference to the fluorescent antibody technique. Apidologie 1973, 4, 331–339.
- Olsen, P.E.; Grant, G.A.; Nelson, D.L.; Rice, W.A. Detection of American foulbrood disease of the honeybee, using a monoclonal antibody specific to Bacillus larvae in an enzyme-linked immunosorbent assay. Can. J. Microbiol. 1990, 36, 732–735.
- Kušar, D.; Papić, B.; Zajc, U.; Zdovc, I.; Golob, M.; Žvokelj, L.; Knific, T.; Avberšek, J.; Ocepek, M.; Ocepek, M.P. Novel TaqMan PCR Assay for the Quantification of Paenibacillus larvae Spores in Bee-Related Samples. Insects 2021, 12, 1034.
- Descamps, T.; De Smet, L.; Stragier, P.; De Vos, P.; de Graaf, D.C. Multiple Locus Variable number of tandem repeat Analysis: A molecular genotyping tool for Paenibacillus larvae. Microb. Biotechnol. 2016, 9, 772–781.
- Bozdeveci, A.; Akpınar, R.; Karaoğlu, A. Isolation, characterization, and comparative genomic analysis of vB_PlaP_SV21, new bacteriophage of Paenibacillus larvae. Virus Res. 2021, 305, 198571.
- Erban, T.; Ledvinka, O.; Kamler, M.; Nesvorna, M.; Hortova, B.; Tyl, J.; Titera, D.; Markovic, M.; Hubert, J. Honeybee (Apis mellifera)-associated bacterial community affected by American foulbrood: Detection of Paenibacillus larvae via microbiome analysis. Sci. Rep. 2017, 7, 5084.
- Schäfer, M.O.; Genersch, E.; Fünfhaus, A.; Poppinga, L.; Formella, N.; Bettin, B.; Karger, A. Rapid identification of differentially virulent genotypes of Paenibacillus larvae, the causative organism of American foulbrood of honey bees, by whole cell MALDI-TOF mass spectrometry. Vet. Microbiol. 2014, 170, 291–297.
- Beims, H.; Janke, M.; Von der Ohe, W.; Steinert, M. Rapid identification and genotyping of the honeybee pathogen Paenibacillus larvae by combining culturing and multiplex quantitative PCR. Open Vet. J. 2020, 10, 53–58.
- Crudele, S.; Ricchiuti, L.; Ruberto, A.; Rossi, F. Quantitative PCR (qPCR) vs culture-dependent detection to assess honey contamination by Paenibacillus larvae. J. Apic. Res. 2020, 59, 218–222.
- Dobbelare, W.; De Graaf, D.C.; Peeters, J.E.; Jacobs, F.J. Development of a fast and reliable diagnostic method for American foulbrood disease (Paenibacillus larvae subsp. larvae) using a 16S rRNA gene based PCR. Apidologie 2001, 32, 363–370.
- Santos, S.B.; Oliveira, A.; Melo, L.D.R.; Azeredo, J. Identification of the first endolysin Cell Binding Domain (CBD) targeting Paenibacillus larvae. Sci. Rep. 2019, 9, 2568.
- Yones, M.; Ma’Moun, S.A.; Farag, R.M.; El-Raouf, M.A. Hyperspectral application for early diagnosis of American foulbrood disease in the honeybee (Apis mellifera L.) larvae. Egypt. J. Remote Sens. Space Sci. 2019, 22, 271–277.
- Anonymous. OIE Terrestrial Animal Health Code. Apidae. Terrestrial Code Online Access. Section 9. Chapter 9.2. 2022. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/ (accessed on 3 January 2023).
- Shimanuki, H.; Knox, D.A. Diagnosis of Honey Bee Diseases. In Agriculture Handbook; No. 69061; U.S. Department of Agriculture: Washington, DC, USA, 2000.
- Bargańska, Ż.; Namiesnik, J.; Ślebioda, M. Determination of antibiotic residues in honey. TrAC Trends Anal. Chem. 2011, 30, 1035–1041.
- Mezher, Z.; Bubnic, J.; Condoleo, R.; Jannoni-Sebastianini, F.; Leto, A.; Proscia, F.; Formato, G. Conducting an international, exploratory survey to sollect data on honey bee disease management and control. Appl. Sci. 2021, 11, 7311.
- Andrade, V.D.M.; Flores, J.L.H.; López, M.A.R.; Hernández, A.C.; Gómez, S.R.; Calvillo, R.P.M.; Martínez, A.G.E.; Pérez, J.C.; Hernández, I.A.; Hidalgo, E.; et al. Evaluation of the presence of Paenibacillus larvae in commercial bee pollen using PCR amplification of the gene for tRNACys. Braz. J. Microbiol. 2019, 50, 471–480.
- Codex Alimentarius. Codex Standard for Honey Codex Stan 12-1981 Council Directive 2001/110/EC; Codex Alimentarius Commission: Rome, Italy, 2001.
- Matović, K.; Ćirić, J.; Kaljević, V.; Nedić, N.; Jevtić, G.; Vasković, N.; Baltić, M.Ž. Physicochemical parameters and microbiological status of honey produced in an urban environment in Serbia. Environ. Sci. Pollut. Res. 2018, 25, 14148–14157.
- Neov, B.; Georgieva, A.; Shumkova, R.; Radoslavov, G.; Hristov, P. Biotic and Abiotic Factors Associated with Colonies Mortalities of Managed Honey Bee (Apis mellifera). Diversity 2019, 11, 237.
- Dickel, F.; Bos, N.M.P.; Hughes, H.; Martín-Hernández, R.; Higes, M.; Kleiser, A.; Freitak, D. The oral vaccination with Paenibacillus larvae bacterin can decrease susceptibility to American Foulbrood infection in honey bees—A safety and efficacy study. Front. Veter-Sci. 2022, 9, 946237.
- USDA Licenses Honeybee Vaccine to Dalan Animal Health. Available online: https://federallabs.org/flc-highlights/federal-lab-news/usda-licenses-honeybee-vaccine-to-dalan-animal-health (accessed on 3 January 2023).
More
