Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Syed Hadi Hasan and Version 2 by Peter Tang.
Carbon quantum dots (CQDs), also known as carbon dots (CDs), are novel zero-dimensional fluorescent carbon-based nanomaterials. CQDs have attracted enormous attention around the world because of their excellent optical properties as well as water solubility, biocompatibility, low toxicity, eco-friendliness, and simple synthesis routes. CQDs have numerous applications in bioimaging, biosensing, chemical sensing, nanomedicine, solar cells, drug delivery, and light-emitting diodes.
- carbon quantum dots
- synthetic methods
- fluorescence
- optical properties
1. Introduction
Recently, carbon-based nanomaterials such as graphene [1], fullerenes [2], nanodiamonds [3], carbon nanotubes (CNTs) [4], and carbon quantum dots (CQDs) have attracted great attention because of their distinctive structural dimensions, as well as their outstanding chemical and physical properties [5]. It was found that the preparation and separation of nanodiamonds are complicated, while other nanomaterials such as graphene, fullerenes, and CNTs do not display good water solubility and also do not exhibit strong fluorescence in the visible region. These limitations prevent their applications in different areas [6]. Although semiconductor quantum dots (SQDs) exhibit good fluorescence properties, because of the presence of heavy metals, they are toxic in nature. This prevents their biological applicationin biosensors, bio-imaging, and drug delivery. In contrast, fluorescent CQDs are nontoxic and, thus, have attracted enormous interest over other carbon-based nanomaterials [7]. Xu et al. in 2004 accidentally discovered CQDs using gel electrophoresis during the purification of single-walled carbon nanotubes [8]. However, the name CQDs was given by Sun et al. in 2006 during the synthesis of carbon nanomaterials of different sizes [9]. Subsequently, CQDs became rising stars among various carbon-based nanoparticles and are considered an extremely precious asset of nanotechnology. CQDs are also known as carbon nano-lights because of their strong luminescence properties [10]. CQDs have attractive features such as ease of synthesis, good water solubility, high photostability, high photoresponse, low cytotoxicity, facile surface functionalization, good catalysis properties, and tunable excitation–emission [11][12][13][14][15][16][17][11,12,13,14,15,16,17]. Due to these characteristic properties, CQDs are widely utilized in photovoltaic devices, medical diagnosis, sensing, drug delivery, catalysts, photocatalysis, optronic devices, bio-imaging, laser, single electron transistors, solar cells, and LEDs [18][19][20][21][22][23][24][25][26][27][28][29][18,19,20,21,22,23,24,25,26,27,28,29]. However, very few reports have been investigated regarding the application of CQDs as a catalyst in photochemical water splitting [30] the preparation of substituted 4H pyran with indole moieties [31], azide-alkyne cycloadditions [32], the degradation of levofloxacin [33], the selective oxidation of alcohols to aldehydes [34], the removal of Rhodamine B [35], the selective oxidation of amines and imine [36], high-efficiency cyclohexane oxidation [37], H-bond catalysis in Aldol condensations [38], intrinsic peroxidase-mimetic enzyme activity [39], and the ring opening of epoxides [40].

2. Synthesis Approach
Since the discovery of carbon quantum dots (CQDs), several convenient, cost-effective, size-controlled, and large-scale production approaches have been developed. For the synthesis of CQDs, two general categories, top-down and bottom-up, approaches are utilized (Figure 1). Even though CQDs synthesis is facile, there are definite challenges related to their synthesis, such as an aggregation of nanomaterials, the tuning of surface properties, and controlling the size and uniformity [41]. To adjust the functional groups present on the surface and achieve better CQDs performance, post-treatment can be conducted in both approaches. Quantum yields (QYs) of CQDs can be enhanced after surface passivation, which eliminates the emissive traps from the surface. CQDs doped with heteroatoms (N and P) or metals such as Au or Mg improve solubility and electrical conductivity [42]. Even though for the synthesis of CQDs, both the top-down and bottom-up approaches have been used, the environmentally and cost-effective bottom-up approach is most commonly used [43].
Figure 1.
The typical approaches for the synthesis of CQDs.
2.1. Top-Down Approach
In a top-down approach, the larger carbon resources such as carbon nanotubes, fullerene, graphite, graphene, carbon soot, activated carbon, etc., are broken down into smaller constituents with the help of different techniques such as laser ablation and electrochemical and arch discharge [28][44][45][46][47][28,44,45,46,47]. Carbon structures with sp2 hybridization that lack efficient energy gaps or band gaps are commonly used as starting materials for top-down processes. Although the top-down approach is extremely helpful and suitable for microsystem industries, it has some limitations, such as the fact that pure nanomaterials cannot be obtained from the large carbon precursor; their purification is costly and also unable to accurately control the morphology and size distribution of CQDs [48].2.2. Bottom-Up Approach
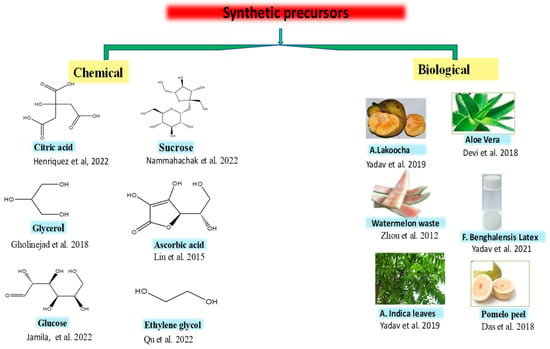
In a bottom-up approach, the smaller carbon resources such as amino acids, polymers, carbohydrates, and waste materials combine to form CQDs by a variety of techniques such as hydrothermal/solvothermal, combustion, pyrolysis, and microwave irradiation. In this method, the size and structure of CQDs depend on a variety of factors such as solvent, precursor molecular structures, and conditions of the reaction (temperature, pressure, reaction time, etc.). The conditions of the reaction are necessary, since they influence the reactants and the extremely casual nucleation and escalation procedure of CQDs. This approach strengthens the material chemistry because of its ease of operation, lower cost, and easier implementation for production in a large scale [49][61]. The precursor used for the synthesis of CQDs may be both chemical and biological, i.e., natural. The chemical precursors include glucose, sucrose, citric acid, lactic acid, ascorbic acid, glycerol, ethylene glycol, etc. [50][51][52][53][54][55][56][62,63,64,65,66,67,68]. The natural sources include Artocarpous lakoocha seeds, rice husks, Azadirachta indica leaves, pomelo peel, the latex of Ficus benghalensis, aloe vera, etc. (Figure 2) [57][58][59][60][69,70,71,72].
Figure 2. Chemical and biological precursors utilized for the synthesis of CQDs [49][50][51][52][53][54][55][56][57][58][59][60].
Chemical and biological precursors utilized for the synthesis of CQDs [61,62,63,64,65,66,67,68,69,70,71,72].
3. Structure of CQDs
Tang et al. reported that CQDs have core–shell structures which are either amorphous (mixed sp2/sp3) or graphitic crystalline (sp2), depending upon the extent of the occurrence of sp2 carbon in the core [61][93]. Graphitic crystalline (sp2) cores were reported by several researchers [62][63][64][94,95,96]. The size of cores is very small (2–3 nm), with a characteristic lattice spacing of ~0.2 nm [65][97]. The cores are categorized depending on the technique utilized for the synthesis and the precursors used, as well as other synthetic parameters (such as duration, temperature, pH, etc.) [66][98]. Generally, the graphitization (sp2) structure is obtained at over 300 °C reaction temperatures, while amorphous cores are obtained at lower temperatures, unless sp2/sp3-hybridized C is present in the precursor [67][99]. To determine the core structure of CQDs, various instrumental techniques such as Transmission Electron Microscopy (TEM) or High Resolution (HR) TEM, Scanning Electron Microscopy (SEM), Raman spectroscopy, and X-ray diffraction (XRD) are utilized. To measure the size and morphology of the CQDs, TEM or SEM are carried out [68][100]. The selected area electron diffraction (SAED) patterns reveal the amorphous or crystalline nature of CQDs [69][101]. The XRD pattern also determines the crystal structure of CQDs. The broad peak at 2θ 23° indicates the amorphous nature of CQD, while the occurrence of two broad peaks at 2θ 25° and 44° specifies a low-graphitic carbon structure analogous to (002) and (100) diffraction [70][102]. The general structure and presence of different functional groups on the surface of CQDs are determined using Fourier transform infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS), elemental analysis (EA), and nuclear magnetic resonance (NMR) [71][72][103,104]. Using nitrogen sorption analysis, the surface area of the carbon nanoparticles is calculated [71][103]. To decide the optical properties and qualitative information regarding the presence of C=C and C=O in CQDs, UV-Vis absorption spectroscopy is carried out [73][105]. To determine the positive or negative charge on the surface of CQDs and the extent of the electrostatic interaction between them, zeta potential is conceded [74][75][106,107].
Figure 3 is the typical structure of carbon quantum dots (CQDs), which reveals the presence of different functional groups (such as carbonyl, carboxyl, hydroxyl, amino, etc.) on the surface of CQDs. The presence of these functional groups was confirmed by instrumental techniques such as FTIR and XPS [76][108].
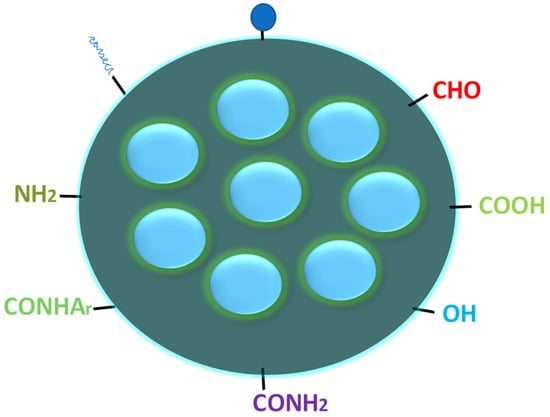

Figure 3.
Typical structure of CQDs with different functional groups on the surface.
4. Optical Properties of Carbon Quantum Dots (CQDs)
4.1. Absorbance
CQDs generally exhibit two absorption bands in the visible region around 280 nm and 350 nm, alongside a tail broadly in the UV region. Hu et al. reported that an absorption band at 280 nm is due to a pi-pi* (π-π*) transition of a C=C bond, and the one at 350 nm is due to an n-π* transition of the C=O bond [77][109]. Figure 4 is the typical UV-visible absorption spectrum of fluorescent CQDs. The absorption properties of CQDs can be influenced by surface modification or surface passivation [78][79][80][81][110,111,112,113]. Depending on the raw precursor and synthesis methodology, the positions of these absorption bands are different to some extent. Doping in CQDs can also alter the absorption wavelength.
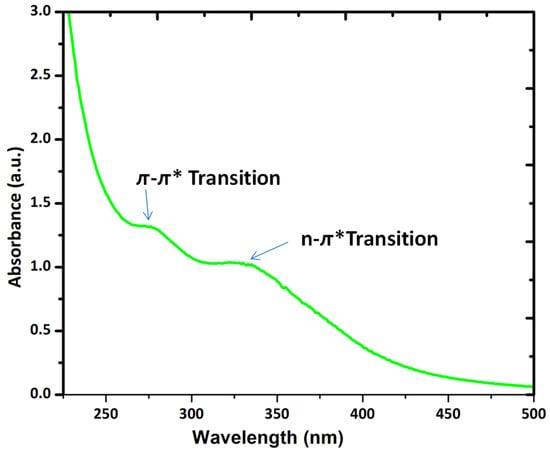

Figure 4.
The UV-visible absorption spectrum of fluorescent CQDs.
The optical properties of CQDs can be customized by doping/co-doping with heteroatoms, functional groups, and surface passivation [82][114]. In the process of surface passivation, a slim insulating (protecting) layer of covering materials such as thiols, thionyl chloride, spiropyrans, and oligomers (polyethylene glycol (PEG), etc.) is formed on the CQDs surface. The important functions of such types of protective layers are to shield CQDs from the adhesion of impurities and to provide stability [83][115]. CQDs with surface-passivating agents become extremely optically active, demonstrating considerable fluorescence from the visible to the near-IR region [84][116]. The quantum yields (QYs) of CQDs can also be enhanced up to 55–60% by surface passivation [82][114]. The absorbance of CQDs improved to longer wavelengths (350–550 nm) after surface passivation with 4,7,10-trioxa-1,13-tridecanediamine (TTDDA) [85][117]. Particle size is associated with the absorption wavelength. As the size of the CQDs increases, absorption wavelength also increases [86][87][118,119]. The CQDs are viable for covalent bonding with functionalizing agents [82][114]. Different functional groups such as amines, carboxyl, hydroxyl, carbonyl, etc., were introduced on the surface of CQDs by surface functionalization. The functionalized CQDs revealed good biocompatibility, high stability, outstanding photoreversibility, and low toxicity compared to undoped CQDs. The efficient technique to modify the CQDs absorption spectrum is doping/co-doping with heteroatoms (such as boron (B), nitrogen (N), fluorine (F), phosphorous (P), and sulfur (S)). The dopant adjusts the bandgap, electronic structure, and, consequently, the optical properties of CQDs by altering the π-π* energy level (related through the core-sp2 carbon system) [88][120]. On increasing N-dopant concentration, a gradual increase in the band gap of the CQDs from 2.2 to 2.7 eV was observed [89][121]. In contrast, it was also found that the doping of N in CQDs results in a reduction in size [90][122]. The CQDs established innovative electronic states, resulting in a reduction in the bandgap of CQDs (about ~48–57%) [91][123]. Zuo et al. synthesized F-doped CQDs using a hydrothermal method which exhibited higher QYs and enhanced the electron transfer and acted as a superior photocatalyst [92][124].
4.2. Photoluminescence
The emission of light from a substance upon the absorption of light (photon) is called photoluminescence (PL). Photoluminescence includes two types, namely fluorescence and phosphorescence. Fluorescent materials emit absorbed light from the lowest singlet excited state (S1) to the singlet ground state (S0). This process is very fast and has a nanosecond lifetime. The transitions that occur among two electronic states in the fluorescence process are allowed because it has the same spin multiplicity. In contrast, in phosphorescence, the transition occurs from the lowest triplet excited state (T1) to singlet ground state (S0), i.e., a forbidden transition occurs according to the spin selection rule.
4.2.1. Fluorescence
The fluorescence properties of CQDs have attracted great attention among researchers because of their several sensing and analytical applications. Numerous mechanisms have been reported to gain deep insight into the cause of fluorescence in CQDs [93][94][95][96][97][98][125,126,127,128,129,130]. Among them, the following two have been found more prominent. The first is that the fluorescence mechanism is due to band gaps’ transitions arising from the π-conjugated domains (sp2-hybridized), which is similar to aromatic molecules employing definite energy band gaps in favor of absorptions and emissions [99][131]. The second cause of fluorescence is related to the surface defects, quantum size effect, carbon core state, surface passivation/functionalization effect, and different emissive traps on the surface of CQDs [100][101][102][132,133,134].
The main reason for the surface defects in CQDs is an unsymmetrical allocation of sp2- and sp3-hybridized carbon atoms, and the existence of heteroatoms such as B, N, P, and S [94][103][126,135]. When this surface defect is independently incorporated into the solid host, it creates surroundings similar to aromatic molecules. These molecules can attract UV light and display various color emissions [99][104][131,136]. CQDs show two types of emission, i.e., excitation-dependent emission (tunable emission) and excitation-independent emission. The tunable emission is due to the presence of various emission sites on the surface of CQDs along with particle size distribution; because of this, most CQDs exhibit tunable emissions [105][137]. The excitation-independent emission is due to the extremely ordered graphitic structure of CQDs [86][118]. CQDs exhibit extensive and unremitting excitation spectra which are highly photostable and have steady fluorescence, in contrast to traditional organic dye [63][106][107][95,138,139].
4.2.2. Phosphorescence
In CQDs, the phosphorescence property is also observed, which was first described by De et al. via dispersing CQDs to polyvinyl alcohol matrix at RT and exciting them with ultraviolet light. The maximum emission obtained was 500 nm, with an average lifetime of 380 ns at a 325 nm excitation [108][140]. Phosphorescence in CQDs arises when the singlet and triplet states of an aromatic carbonyl group in CQDs and polyvinyl alcohol matrix are close in energy to assist spin–orbit coupling, which increases the intersystem crossing (ISC). By using microwave synthesis, Lu et al. synthesized ultra-long phosphorescent carbon quantum dots (P-CQDs). When P-CQDs were excited at 354 nm, they displayed yellow-green phosphorescence (525 nm) for up to 9 s. They concluded that as the pH increases, the phosphorescence intensity of P-CQDs gradually decreases. The reason is that protonation dissociates the hydrogen bonds and distresses the phosphorescent sources. By introducing the tetracyclines (TCs), the phosphorescence of P-CQDs was quenched. They applied P-CQDs as biological and chemical sensing and time-resolved imaging [109][141]. Figure 5 is the typical excitation (black line) and emission (red line) spectrum of fluorescent CQDs.
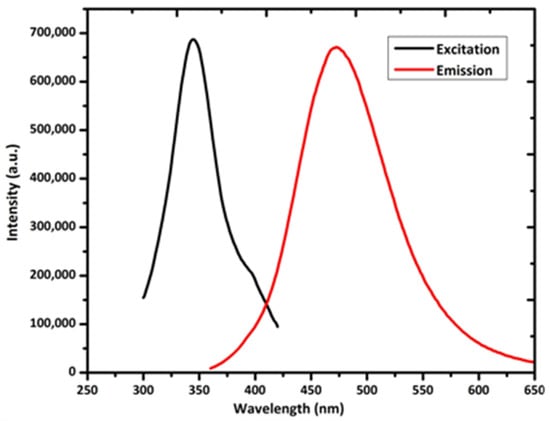

Figure 5.
Excitation and emission spectrum of CQDs.
