You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Heidi Makrinioti and Version 2 by Catherine Yang.
Obesity-related asthma is a heterogeneous childhood asthma phenotype with rising prevalence. Observational studies identify early-life obesity or weight gain as risk factors for childhood asthma development. Oxidative stress is a central pathogenetic process in obesity-related childhood asthma and is considered to drive other metabolic processes, including dysregulation of fatty acids, peripheral blood ketones, amino acid metabolism, and insulin resistance.
- obesity
- asthma
- subtyping
- metabolomics
- endotypes
1. Oxidative Stress and Dysregulation of Free Fatty Acid Metabolism Pathways in Childhood Asthma
The pathway associating IFNAR signaling and free fatty acid metabolism in obese children may be important. The role of free fatty acids has been reported in several metabolomic studies in childhood asthma [1][2][23,25]. However, there is still controversy in regard to the associations with long-term outcomes (i.e., asthma) [3][4][10,84]. Till now, the evidence shows that decreased peripheral blood n-3 long-chain fatty acid levels are associated with an increased risk for obesity-related asthma [5][85]. However, sparse evidence shows that interventions with n-6 long-chain fatty acids may be associated with increased airway inflammation [6][7][86,87]. These ostensibly controversial findings can be possibly explained by the timing, sensitivity of measurement tools, or possible interactions with other measured metabolites but need to be further elucidated.
In contrast to the evidence around the role of polyunsaturated long-chain fatty acids, data from observational studies and experimental models investigating the role of short-chain fatty acids point consistently toward a protective role in childhood asthma [8][88]. However, evidence around their role in obesity-related childhood asthma is missing. Short-chain fatty acids are synthesized by gut microbiota through the fermentation of undigested carbohydrates and dietary fibers in the gastrointestinal tract [9][89]. Observational studies show that decreased levels of short-chain fatty acids are associated with increased asthma severity [3][10]. Additionally, reduced secretion of short-chain fatty acids (e.g., acetate, propionate, and butyrate) secondary to gut microbiome changes is associated with an increased risk of childhood asthma development [10][90]. Although the protective role of short-chain fatty acid supplementation is yet to be confirmed, mouse models of short-chain fatty acid supplementation show a protective effect on asthma development in the offspring [11][91]. These findings are also supported by observational data associating a reduced fecal level of short-chain fatty acids during pregnancy with an increased risk of asthma development. Recent in vivo pre-clinical data also indicate protection against rhinovirus-induced respiratory tract infections (i.e., the most common trigger of asthma exacerbations) following intranasal administration of short-chain fatty acids. However, this study does not focus on obesity-driven airway inflammation [12][92].
2. Oxidative Stress and Peripheral Blood Ketones and Glucose Dysregulation in Obesity-Related Asthma
In addition to fatty acid metabolism pathways, there are observational studies that associate blood ketone levels, amino acid metabolism, and control of hepatic glucose release with obesity-related asthma development in children and adults [13][14][93,94]. More specifically, past observational studies have shown that increased energy demands and hypoxia (i.e., in asthma) are associated with an increase in the consumption of ketone bodies [2][25]. There are two main ketone bodies, acetoacetate, and beta-hydroxybutyrate, being detected at lower levels in obese children with asthma compared to controls.
In regard to amino acid metabolism, lower levels of plasma histidine and glutamine have been associated with increased asthma incidence [2][25]. Histidine is an essential amino acid [15][95]. Lower histidine levels have also been detected in obese women without asthma [16][96]. In female animal models, supplementation with histidine has been shown to impact weight increase and improve airway inflammation and oxidative inflammatory responses driven by adipose tissue [17][97].
In addition, a higher prevalence of insulin resistance and consequent hyperglycemia has been reported in obese asthmatic children [13][93]. Past studies have shown that impaired insulin secretion during the first years of life has been related to an increased risk of asthma development [18][98]. Several studies report that insulin resistance, in combination with other factors (e.g., abdominal fat and metabolic syndrome) [13][93], is associated with reduced lung function in asthmatic and non-asthmatic children. Interestingly, these studies in the general adult population show that insulin resistance is associated with an obstructive rather than restrictive (i.e., obesity-related) decline in lung function.
The pathophysiologic mechanisms underlying these findings are poorly understood. Mechanistic studies have shown that airway smooth muscle (ASM) cells express insulin receptors and develop a pro-contractile phenotype when exposed to high levels of insulin [19][99]. Based on these findings, the reported associations between insulin resistance and asthma may be enhanced by obesity and that glucose regulation pathways may help subtyping obesity-related asthma in childhood.
In conclusion, metabolic pathways may explain a large part of obesity-related asthma pathophysiology in children [1][2][20][21][22][23][23,25,41,42,65,100]. Although levels of other metabolites, including urinary prostaglandin D2 and urinary leukotriene E4, have been associated with increased prevalence of childhood asthma, there is no evidence related to associations with obesity-related asthma prevalence [24][101]. The metabolomics studies that have reported associations between childhood and adult asthma and obesity are depicted in Table 1, and the main metabolic profiles are shown in Figure 12. Both fatty acid metabolism and glucose regulation pathways merit further investigation, ideally in integration with other “omic” data so that asthma endotypes can be better described.
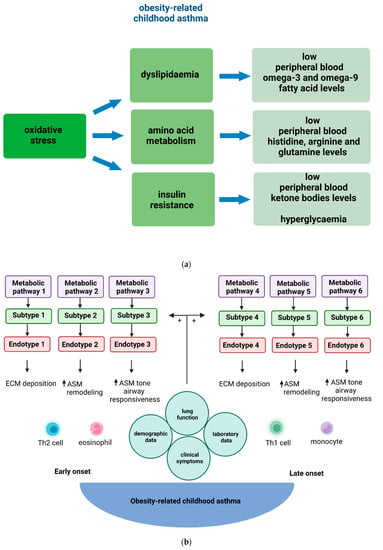
Figure 12. (a) Main metabolic profiles described as more prevalent in obese children with asthma in comparison to the control group (i.e., non-obese children with asthma). (b) The obesity-related childhood asthma phenotype consists of various endotypes. In children, early onset of obesity-related asthma is usually associated with TH2-high inflammation, whilst late onset of obesity-related asthma is associated with TH2-low inflammation. Metabolic dysregulation contributes to both TH2-high and TH2-low systemic and airway inflammation that underlies ECM deposition, ASM proliferation, related airway tissue remodeling, and airway reactivity in obesity-related asthma endotypes in children. Metabolomic pathways, identified by metabolomic strategies, provide further insight into the pathogenetic mechanisms underlying these diverse endotypes. Subtypes of obesity-related childhood asthma derive through the combination of clinical information with endotypic characterization data. Obesity-related asthma subtypes refer to groups of children with common clinical characteristics and endo-metabotypes (meaning a subtype of an endotype described by similar metabolic dysregulation mechanisms). Abbreviations: ASM: Airway smooth muscle, ECM: Extracellular matrix, TH2: T helper 2.
Table 1.
Table describing metabolomics studies that have reported associations between childhood and adult asthma and obesity.
| Citation (Country) |
Study Type | Study Population and Size |
Exposure(s) or Intervention(s) |
Metabolomics Technique | Cellular Location of Metabolic Pathway | Outcome (Incident Asthma or Prevalent Asthma or Asthma Control) |
Measure of Association |
|---|---|---|---|---|---|---|---|
| studies in children | |||||||
| Qu H.Q. et al. [2][25] United States |
case-control study | 603 children with asthma (stratification through weight level) and 593 children without asthma | peripheral blood samples ketone bodies, histidine, glutamine, saturated fatty acids, very low-density lipoprotein |
NMR spectroscopy | endoplasmic reticulum, nucleus, cytoplasm, mitochondria | prevalent asthma by 18 years | lower plasma levels of histidine and glutamine more prevalent in asthma cases than in controls (p < 0.05) low peripheral blood ratio of ketone bodies, citrate, and fatty acids more prevalent in asthma cases with increased weight than in controls (p < 0.01) |
| Rastogi G. et al. [20][41] United States |
case-control study | 82 obese adolescents and 86 non-obese adolescents | peripheral blood samples insulin, lipids, adipokines |
NMR spectroscopy | endoplasmic reticulum, cytoplasm, mitochondria | prevalent asthma by 16 years | increased adipokines and lipids levels are independently associated with reduced lung function and asthma prevalence by 16 years |
| Thompson D. et al. [22][65] United States |
cohort study analytical study |
26 children with obese asthma phenotype and 28 children with non-obese asthma phenotype | serum glucose, insulin, lipids, fatty acid levels and TH cell transcriptome neck, WHR, and BMI z score |
NMR spectroscopy | endoplasmic reticulum, nucleus, and mitochondria | asthma control | decreased lipids and increased fatty acid levels associated with increased asthma control and improved pulmonary function in obese children than in non-obese controls (p < 0.05) |
| Fitzpatrick A.M. et al. [25][83] United States |
case-control study | 257 children with asthma | peripheral blood samples leptin, adiponectin, C-reactive protein, total cholesterol, IL-1β, IL-6, IL-17, interferon gamma, tumor necrosis factor alpha, monocyte-chemoattractant protein-1, and amino acid metabolites |
NMR spectroscopy | endoplasmic reticulum, cytoplasm, mitochondria | asthma control (6–17 years old) |
within the group of obese children, lower concentrations of arginine-related metabolites associated with reduced asthma control lower vs higher (p <0.05) |
| Papamichael M.M et al. [26][24] Greece |
case-control study | 64 children with asthma | peripheral blood samples plasma fatty acid metabolites (linoleic, oleic, erucic, cis-11-eicosenoic, arachidic acids, α-linolenic, EPA and DHA) |
GM-CS | endoplasmic reticulum, nucleus, cytoplasm, mitochondria | asthma control (5–12 years old) |
peripheral blood α-linolenic, EPA and DHA levels not associated with reduced asthma control decreased level of linoleic, oleic, erucic, cis-11-eicosenoic, arachidic acids associated with reduced asthma control increased vs non-increased (p <0.05) |
| Tobias T.A.M. et al. [27][102] United States |
case-control study | 39 obese children with asthma, 39 normal weight children with asthma, 38 obese controls and 42 normal weight controls | peripheral blood samples plasma polyunsaturated fatty acids, carotenoids |
NMR | endoplasmic reticulum, nucleus, and mitochondria | asthma control (13–18 years old) |
increased level of peripheral blood polyunsaturated long-chain fatty acids correlated with improved asthma control (p < 0.01) |
| studies in adults | |||||||
| Liu Y. et al. [28][103] China |
case-control study | 11 obese adults with asthma and 22 non-obese adults with asthma | peripheral blood and sputum samples peripheral blood cyanoaminoacid, caffeine, valine, uric acid, N-methy-DL-alanine and beta-glycerophosphoric acid metabolism sputum tryptophan and pentose phosphate metabolism |
GC-MS | endoplasmic reticulum, nucleus, and mitochondria | prevalent asthma by 57 years (18–57 years old) |
decrease in 3-hydroxybutyric acid, linolenic acid, isoleucine in obese vs non-obese adults with asthma (p < 0.05) |
| Rastogi D. et al. [23][100] United States |
case-control study | 334 overweight adults, and 648 obese adults | peripheral blood and sputum samples insulin resistance, HDL levels |
Elisa in peripheral blood samples | endoplasmic reticulum, nucleus, and mitochondria | prevalent asthma by 60 years | high peripheral blood HDL levels associated with prevalent asthma in obese rather than overweight adults (p < 0.05) |
| Maniscalco M. et al. [29][104] Italy |
case-control study | 25 obese adults with asthma and 30 non-obese adults with asthma | EBC samples methane, glyoxylate/dicarboxylate, and pyruvate |
NMR spectroscopy | cytoplasm, nucleus, and mitochondria | prevalent asthma by 50 years (30–50 years old) |
EBC samples from obese patients with asthma had increased glucose, butyrate, and acetoin levels and decreased formate, tyrosine, ethanol, ethylene glycol, methanol, n-valerate, acetate, saturated fatty acids, and propionate levels as compared to non-obese patients with asthma (p < 0.004) |
| S. Y. Liao et al. [30][105] United States |
randomized controlled trial | 19 patients with severe asthma | peripheral blood samples intervention treatment with L-arginine or placebo at 0.05 mg/kg for 12 weeks, then 6-week washout period, and then treatment with L-arginine or placebo at 0.05 mg/kg exposures arginine-related metabolites, GLP-1, insulin |
MS | endoplasmic reticulum, cytoplasm | asthma control (5–12 years old) |
L-arginine supplementation was associated with increased insulin levels and decreased GLP-1 levels between those who received this and the control group (p = 0.02) |
| Mani M.L. et al. [31][106] United States |
case-control study | 19 healthy adults and 34 adults with asthma | peripheral blood samples bile acid levels (glycocholic acid and glycoursodeoxycholic acid) |
LC-MS | endoplasmic reticulum, cytoplasm, and mitochondria | asthma control (18–65 years old) |
increased peripheral blood glycocholic and glycoursodeoxycholic acid levels associated with reduced asthma control (p < 0.05) |
