TACE is widely performed for inoperable HCCs; however, its therapeutic effects on HCC is strongly influenced by techniques. Therefore, it is important to properly use the TACE techniques according to the patient and tumor condition. Among them, cTACE is a skill to cure localized HCCs; however, it should be performed as selectively as possible because it also damages the normal liver.
- hepatocellular carcinoma
- transarterial chemoembolization
- iodized oil
- gelatin sponge particle
- drug-eluting bead
1.
Transarterial chemoembolization (TACE) is a first-line treatment for patients with hepatocellular carcinoma (HCC) in Barcelona Clinic Liver Cancer stage B (BCLC-B). There are two major techniques of TACE: conventional TACE (cTACE) using iodized oil and gelatin sponge particles, and TACE using drug-eluting beads (DEB-TACE). The latest randomized controlled trial proved the superiority of cTACE regarding local effects over DEB-TACE; however, cTACE also damages the liver more severely. Therefore, cTACE should be performed for localized HCCs as selectively as possible. On the other hand, DEB-TACE has less liver toxicity and is favorable for patients with an advanced age, large and/or bilobar tumors, or a poor liver function.
Introduction
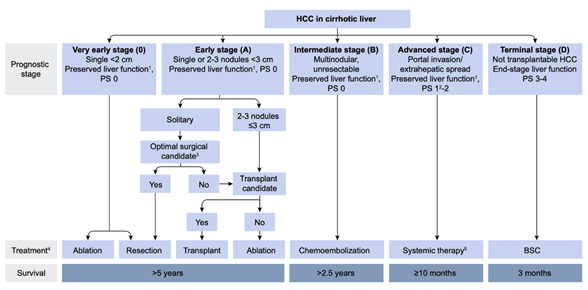
Since the first report by Yamada et al.[1] [1], transarterial chemoembolization (TACE) has been widely performed for inoperable hepatocellular carcinoma (HCC). In 2002, two randomized controlled trials (RCT) demonstrated that TACE for inoperable HCC showed a survival benefit compared with best supportive care (BSC)[2][3] [2,3]. Two meta-analyses also proved the clinical usefulness of TACE in prolonging HCC patients’ survival[4][5] [4,5]. Therefore, TACE has been recognized as an effective treatment option for inoperable HCC worldwide, and as a first-line treatment for HCC in Barcelona Clinic Liver Cancer stage B (BCLC-B) (Figure 1)[6] [6].
Now, two major techniques of TACE have been performed: conventional TACE (cTACE) with iodized oil (Lipiodol 480, Guerbet Japan, Tokyo, Japan) and gelatin sponge (GS) particles; and TACE with drug-eluting beads (DEB-TACE). cTACE has been mainly developed in Asian regions, whereas DEB-TACE has been focused in Western countries. The concepts and rationales for both techniques, as well as adverse effects, are quite different; therefore, we should use them appropriately according to the patient and tumor condition. Thus, the purpose of this article is to describe the TACE strategy for BCLC-B HCC, mainly based on the conceptualistic position and techniques of TACE treatment in Japan.
Figure 1. European Association for the Study of the Liver (EASL) clinical practice guidelines 2018 on hepatocellular carcinoma. Reprinted with permission from Ref.[6] [6]. Abbreviations: PS, performance status; BSC, best supportive care
2. Hemodynamics in the Liver and HCC
Understanding of the hemodynamics in the liver and HCC is very important to perform TACE procedures safely and effectively.
2.1. Microvasculature of the Normal Liver
Two major branches are arising from the terminal hepatic artery: one terminates within the portal tract supplying the bile duct (peribiliary vascular plexus: PBP), portal tract interstitium, and portal vein wall (portal vein vasa vasorum); and the other one penetrates the liver parenchyma unaccompanied by the portal vein or bile duct, named the isolated artery[7] [7]. Most terminal hepatic arterioles connect with the PBP, and blood from the PBP directly drains into the portal venules and hepatic sinusoids. Therefore, bile duct injury can develop following TACE when the PBP is severely damaged[8] [8]. The incidences of bile duct injury are usually high in the normal liver compared with the cirrhotic liver because the PBP is more hypertrophied in the cirrhotic liver[9] [9]. The isolated artery communicates with the hepatic capsular arterial plexus, as well as with extrahepatic arteries, such as the internal mammary and inferior phrenic arteries, through the ligaments in the bare area of the liver. This communication can help the tumor mainly in the bare area of the liver to survive after TACE[10] [10]. The intersegmental collateral formation between different segmental arteries can also develop following TACE and promote the tumor recurrence.
The normal liver parenchyma is supplied by a dual blood supply, including the portal venous system and the hepatic arterial system; thus, embolization of the hepatic artery can theoretically achieve selective ischemic necrosis of the tumor tissues alone. However, hepatic necrosis can also develop when superselective cTACE is performed[11][12][13] [11–13].
2.2. Hemodynamics in Hypervascular HCC
Hemodynamics in the hepatocytic nodules can change according to the grades of malignancy of the nodules. During multistep hepatocarcinogenesis, the intranodular arterial supply initially decreases, but then acutely increases because of the increase in abnormal arterial supply. On the other hand, the intranodular portal supply gradually decreases, and finally disappears in moderately to poorly differentiated HCC tissue[14] [14]. Therefore, overt HCC is predominantly supplied by arterial blood; however, some tumor tissues are still partially supplied by portal blood, such as well-differentiated tumor portions and tumors invading the surrounding liver through the capsule (capsular/extracapsular invasion) mainly located in the periphery of the tumor, as well as microsatellite lesions[15] [15].
Simultaneously, the hepatic veins surrounding HCC are also occluded by tumor invasion and the tumor blood drains into the peritumoral area through the portal vein remnant in the capsule, depicted as corona enhancement on computed tomography (CT) during hepatic arteriography (CTHA)[16][17].
Simultaneously, the hepatic veins surrounding HCC are also occluded by tumor invasion and the tumor blood drains into the peritumoral area through the portal vein remnant in the capsule, depicted as corona enhancement on computed tomography (CT) during hepatic arteriography (CTHA) [16,17].
3. Limitation of TACE and Necessity of Curative TACE
3.1. Limitation of TACE
As mentioned above, well-differentiated tumor portions, capsular and/or extracapsular tumor invasions, and microsatellites are usually supplied by a dual blood supply; therefore, they can survive after simple embolization of the arterial side, particularly when it is performed non-selectively[15][18] [15,18]. Additionally, there are two possible causes of local tumor recurrence after TACE. One important cause is portal blood supply to tumors via the portal venules and surrounding hepatic sinusoids due to the reversal of portal flow through the tumor drainage appearing during blockage of the hepatic arterial flow[18][19] [18,19]. The other cause is arterial collateral supply to tumors from the neighboring arteries, sometimes from the extrahepatic arteries through the communication between the hepatic capsular arterial plexus and isolated artery[10] [10].
3.2. Necessity of Curative TACE
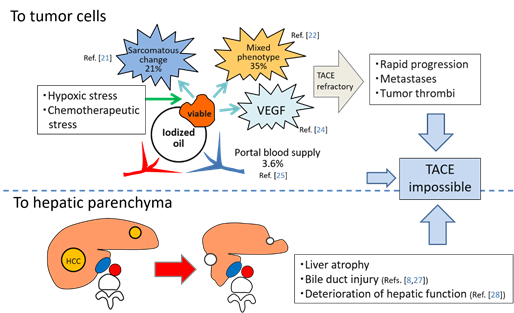
TACE is recognized as a palliative treatment option for HCC; however, it is reported that a complete response (CR) after the initial TACE is the most robust predictor of long-term survival[20] [20]. In other words, ineffective TACE may shorten a patient’s lifespan. TACE loads hypoxic and chemotherapeutic stress on HCC, and some surviving tumors frequently change to more aggressive and TACE-resistant tumor tissues, such as sarcomatous appearances or mixed hepatocholangiocellular phenotypes[21][22] [21,22]. Hypoxia induced by TACE also stimulates vascular endothelial growth factor (VEGF) production by the residual tumor cells, which can promote the tumor progression following TACE[23][24] [23,24]. Furthermore, some surviving tumors can be nourished by the portal venous system if the arterial branches are markedly attenuated by TACE and extrahepatic collaterals cannot develop[25] [25]. These suggest that ineffective TACE can also produce uncontrollable tumors. In our recent analysis, the incidences of intrahepatic distant recurrence (IDR) of HCC in patients with local tumor progression (LTP) were significantly higher than in patients without LTP (p = 0.0004)[26] [26]. This indicates that some IDR may be associated with LTP: metastases may be from locally progressed tumors or may be from newly developed tumors promoted mainly by high expression of VEGF production from the viable tumor cells. In addition, TACE damages the liver and bile ducts[8][27][28] [8,27,28] (Figure 2). Therefore, it is important to perform curative TACE when the tumor is localized and controllable.
Figure 2. Schematic representation of negative aspects of TACE. Abbreviations: VEGF, vascular endothelial growth factor.
4. Technical Tips for Performing Effective Superselective cTACE
4.1. Achievement of Marked Portal Vein Visualization with Iodized Oil during cTACE
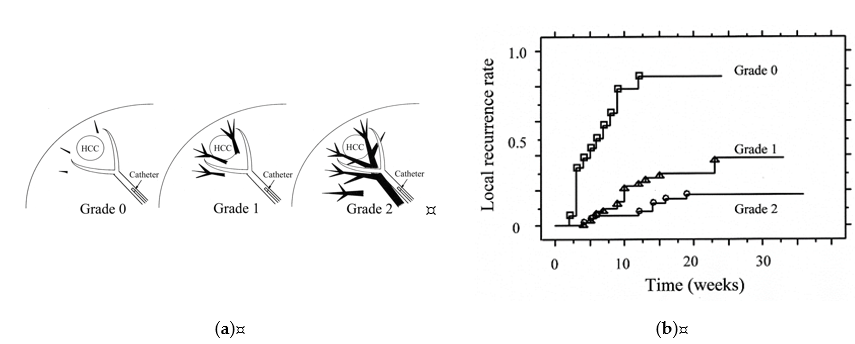
There is a relationship between the grades of portal vein visualization with iodized oil and local tumor recurrence. We divided the grades of portal vein visualization with iodized oil into three grades on spot radiographs obtained during the procedure: grade 0, no visualization; grade 1, visualization adjacent to the tumor; and grade 2, visualization in the whole or extended embolized area, and the local recurrence rates in the grade 2 group were significantly lower than those in the grades 1 and 0 groups (p < 0.0485 and p < 0.0001, respectively) (Figure 4) [29][40].
Figure 4. Classification of grades of portal vein visualization with iodized oil and relationship between local tumor recurrence. (a) Schematic representation of grades of portal vein visualization with iodized oil; (b) Graph shows the local recurrence rates calculated with the Kaplan-Meier method for each portal vein visualization group. The local recurrence rates for tumors in the grade 2 group were significantly lower than those for tumors in the grades 1 and 0 groups (p = 0.0485 and p < 0.0001, respectively). The local recurrence rates for tumors in the grade 1 group were also significantly lower than those in the grade 0 group (p < 0.0001). Reprinted with permission from Ref.[29] [40].
In superselective cTACE, iodized oil should be slowly injected to avoid “oil cast formation” in the arteries. The endpoint of iodized oil injection is grade 1 portal vein visualization, not grade 2, because iodized oil in the tumor and surrounding liver is pushed into the portal vein by GS slurry injection and widely distributes into the whole embolized area, and frequently beyond the embolized area. As a result, grade 2 visualization can be obtained[30] [74]. When the flow of the tumor-feeding branch unexpectedly stops before adequate portal vein visualization, we can adopt two measures: injection of 0.5 μg of prostaglandin E1 or 0.5 mL of 2% lidocaine through the catheter to increase arterial flow; and more distal advancement of a microcatheter to achieve a semi-wedged condition[30] [74]. The latter technique should be attempted first, if possible, because it is usually more effective. When the microcatheter is advanced more distally, iodized oil can be re-injected. In such a condition, iodized oil can flow forward due to the injection force, because stretching the catheterized artery and the mass effect of the microcatheter itself can prevent backflow of iodized oil. As a result, the volume of iodized oil passing through the arterioportal and arterial communications can increase and embolic effects can be enhanced.
4.2. Importance of the Order of Embolization of Each Tumor Feeder
When multiple feeding branches are present, the order of embolization of each feeder is key to improve the therapeutic effect of cTACE. The main tumor feeder should be embolized last because dense retention of iodized oil and contrast material disturbs the depiction of the residual tumor stain and other small feeders on digital subtraction angiography performed immediately after cTACE. Additionally, embolic materials injected into the main tumor feeder are sometimes pushed back by the reversed flow through the minor tumor feeder when the flow of the main tumor feeder becomes near stasis[30] [74]. In tumors partially fed by an extrahepatic collateral supply, the feeding branch of the extrahepatic artery should also be embolized first, if possible, because arterial blood flow to tumors through the extrahepatic artery immediately increases when the hepatic arterial flow is stopped[31] [75]. Moreover, each tumor feeder should also be embolized from distal to proximal in order to avoid inadvertent occlusion of the distal tumor feeders with overflowing embolic materials during embolization of the proximal tumor feeder[30] [74]. However, feeders that are considered difficult to catheterize, such as the caudate artery or tiny tumor feeders arising from extrahepatic arteries, should be embolized first, because the performance of a microcatheter-microwire system is gradually deteriorated during the procedure[30] [74].
4.3. Usefulness of Stepwise cTACE for Large HCCS
cTACE is also widely performed for large HCCs; however, the previously reported 5-year overall survival rate was only 7–9.7%[32][33] [76,77]. In addition, the use of a large volume of iodized oil for large tumors carries the risk of catastrophic complications, such as systemic embolization of the lung or brain[34][35] [78,79] and acute tumor-lysis syndrome[36] [80]. Therefore, we typically perform stepwise superselective cTACE for localized tumors >7 cm[37] [38][81,82]. Two to three cTACE sessions are scheduled depending on the vascular anatomy, and each session is performed in a superselective fashion using ≤10 mL of iodized oil at 3- to 10-week intervals according to the patient and tumor conditions. In our recent analysis of 25 patients with HCC ≥10 cm (mean, 130 ± 27.6 mm [range, 101–193], single [n = 12], 2–9 [n = 6, mean, 4.2 ± 2.6 ], and ≥10 tumors [n = 7, including 2 with ≥50 tumors], with vascular invasion [n = 5]), the 5-year survival rate was 23.1%, and that of patients with tumors ≤3 lesions was 38.9%[38] [82]. This indicates that cTACE is also effective for localized huge HCCs. However, cTACE of extrahepatic collateral arteries was required in 84% of patients during the treatment course[38] [82]. Therefore, the outcomes of cTACE for huge HCC may be more strongly influenced by the TACE technique compared with cTACE for small HCC. Bland embolization with gelatin sponge particles followed by cTACE is another alternative option for huge HCC to safely embolize the tumor[39] [83].