Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Lindsay Dong and Version 2 by Lindsay Dong.
Compared with organic analogs, onium derivatives of nido-carborane have increased stability due to the stabilizing electron-donor action of the boron cage. Charge-compensated derivatives are considered according to the type of heteroatom bonded to a boron atom.
- nido-carborane
- charge compensation
- onium derivatives
1. Introduction
The synthesis of the first polyhedral boranes, carboranes, and metallacarboranes in the early 1960s was one of the major highlights in the development of inorganic chemistry over the last century [1]. The first reports on the synthesis of icosahedral carboranes appeared almost sixty years ago, at the end of 1963 when both the United States and the Soviet Union almost simultaneously declassified documents about their boron fuel projects [2][3][4][5][6]. A few months later, the nucleophile-promoted removal of one boron atom from the icosahedral ortho-carborane cage to form the 11-vertex nido-carborane cage species (Figure 1) was reported [7][8]. It was one of the most significant findings in the early years of the development of carborane chemistry, and now, more than five decades later, it remains indispensable for the synthesis of numerous metallacarboranes [9][10][11][12][13][14][15][16][17] and hydrophilic functionalized carboranes for medical [18][19][20][21][22][23][24][25][26][27] and other [28][29][30][31][32][33][34][35][36][37][38][39][40] applications.
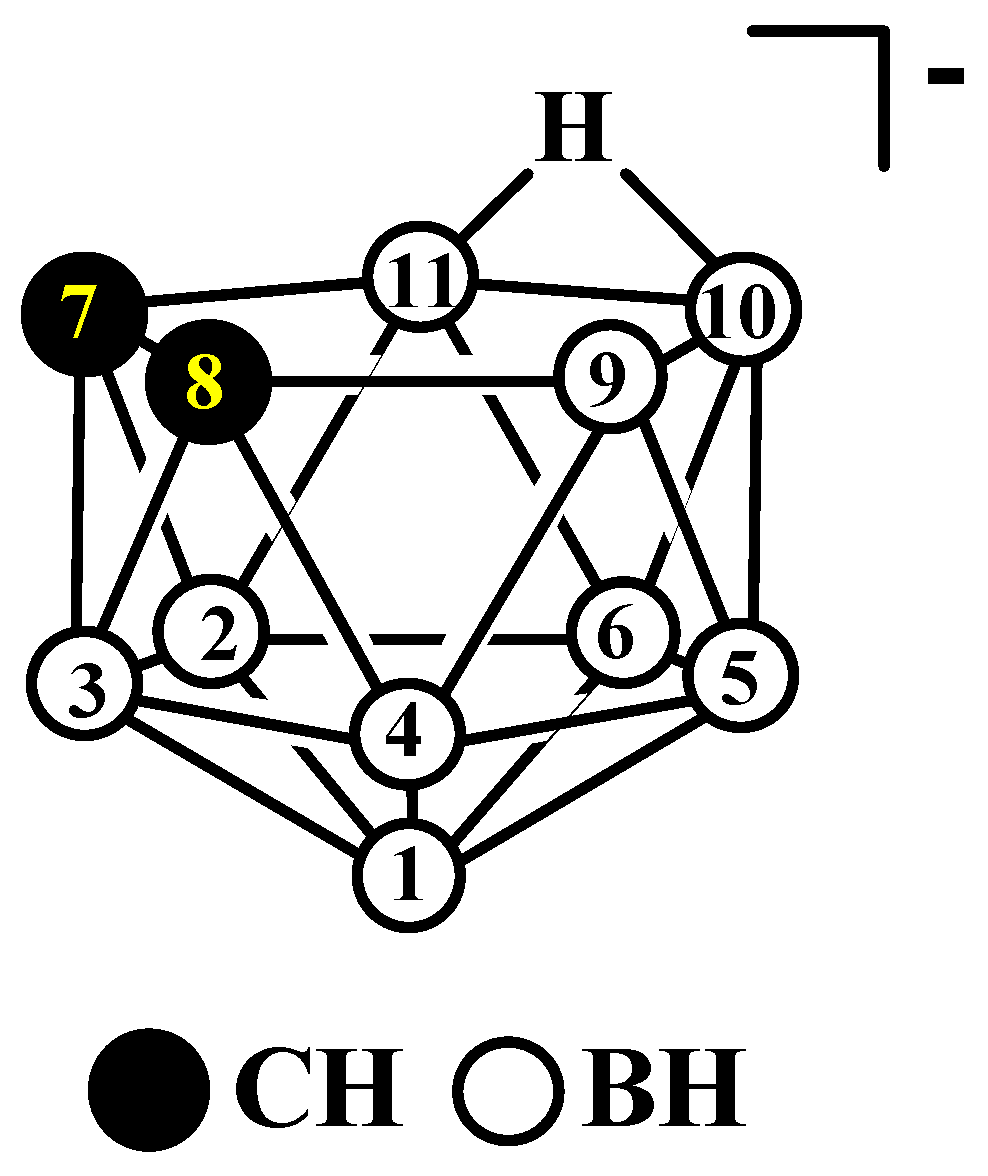
Figure 1. The idealized structure and atom numbering of nido-carborane [7,8-C2B9H12]−.
Metallacarboranes based on the dicarbollide ligand [7,8-C2B9H11]2−, which is formed upon the deprotonation of nido-carborane with strong bases, resemble the well-known transition metal cyclopentadienyl complexes. However, the dicarbollide ligand differs from the cyclopentadienyl ligand in a number of ways. In addition to its 3D character, the dicarbollide ligand is a significantly stronger donor than the cyclopentadienyl one and has a double charge. The donor nature of the dicarbollide ligand can be largely tuned via the introduction of substituents of various natures. At the same time, the charge of the ligand can be partially compensated by introducing into the dicarbollide ligand the so-called charge-compensating substituents of an onium nature (ammonium, phosphonium, sulfonium, etc.). This significantly brings the properties of the dicarbollide and cyclopentadienyl complexes closer together and causes a high interest in metallacarboranes based on charge-compensated dicarbollide ligands [41][42][43][44][45][46][47][48][49][50].
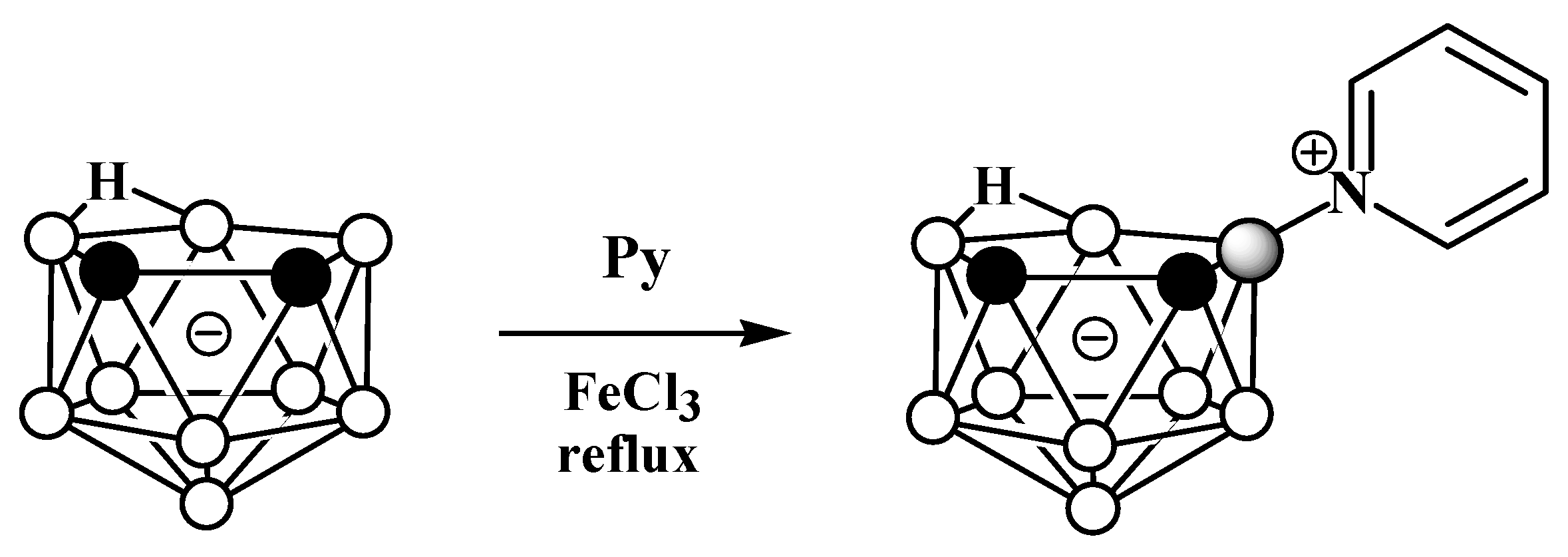
2. Charge-Compensated Derivatives of Nido-Carborane with Boron–Nitrogen Bond
Due to the great diversity of nitrogen chemistry, the charge-compensated derivatives of nido-carborane with the B-N bond are characterized by the greatest variety of forms. The first example of the synthesis of charge-compensated derivatives of nido-carborane with a B-N bond was the reaction of the parent nido-carborane with pyridine in benzene in the presence of anhydrous FeCl3, leading to the asymmetrically substituted pyridinium derivative 9-Py-7,8-C2B9H11 (Scheme 1) [51], the structure of which was later supported via a single-crystal X-ray diffraction study (Figure 2) [52]. When FeCl3·6H2O was used instead of anhydrous FeCl3, the by-product of the reaction was the disubstituted pyridinium derivative 9,11-Py2-7,8-C2B9H9 [52]. The reaction with 7,8-dimethyl-nido-carborane proceeds in a similar way, giving 9-Py-7,8-Me2-7,8-C2B9H9 [51]. In a similar way, the reaction of nido-carborane with methyl isonicotinate in the presence of FeCl3 in refluxing benzene results in 9-(4′-MeO(O)CC5H3N)-7,8-C2B9H11 [53].
Scheme 1. The synthetic process to obtain 9-Py-7,8-C2B9H11.

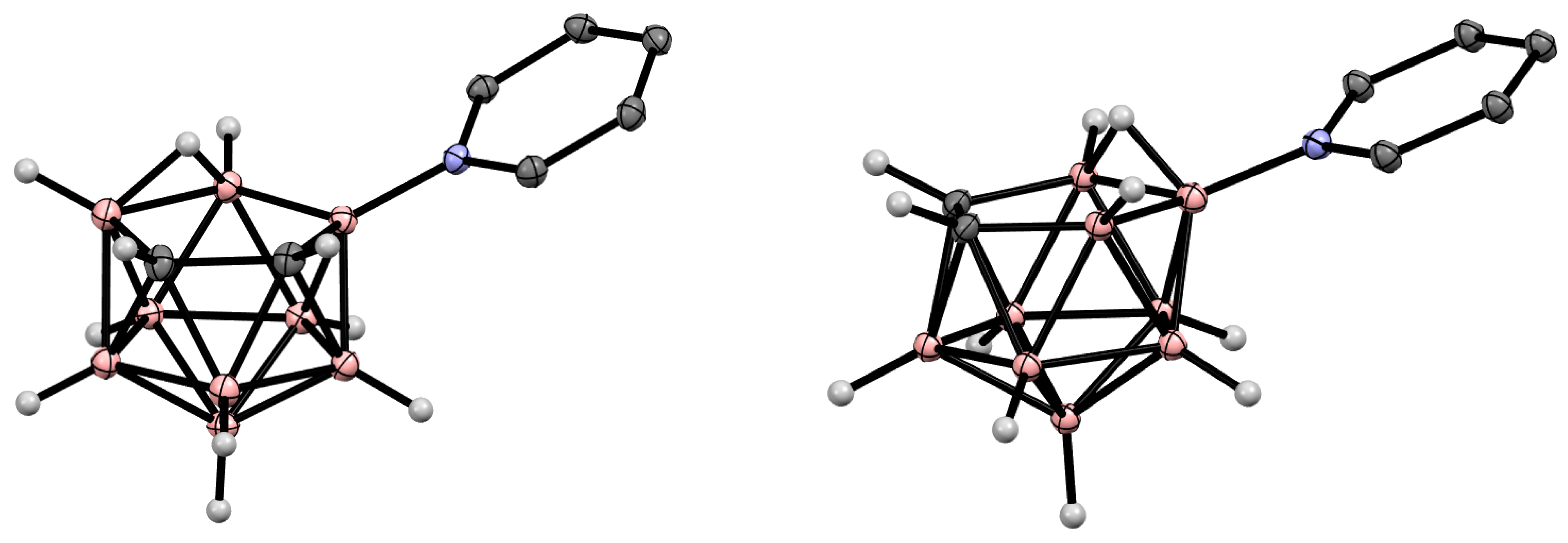
Figure 2. Crystal molecular structures of 9-Py-7,8-C2B9H11 (left) and 10-Py-7,8-C2B9H11 (right). Hydrogen atoms of organic substituents are omitted for clarity.
The reaction of nido-carborane with pyridine in the presence of HgCl2 in refluxing benzene gives a mixture of the symmetrically and asymmetrically substituted pyridinium derivatives 10-Py-7,8-C2B9H11 and 9-Py-7,8-C2B9H11 in a ratio of 2:1 (Scheme 2) [50][54]. The reaction of 7,8-dimethyl-nido-carborane [7,8-Me2-7,8-C2B9H10]− with pyridine proceeds in a similar way [54].
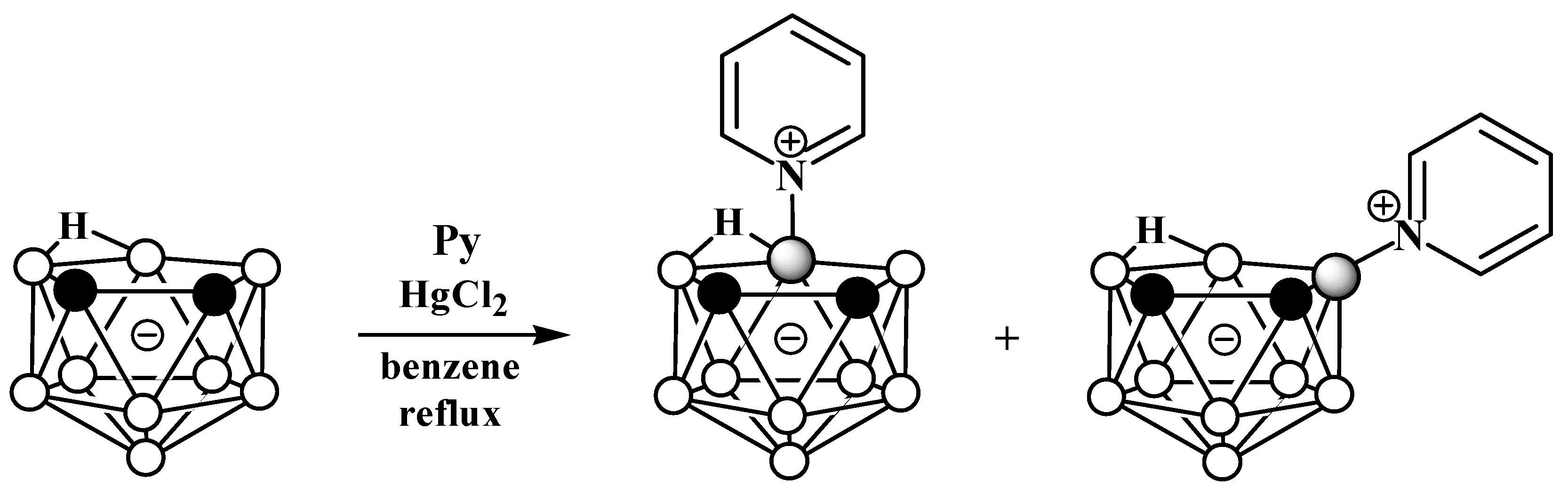

Scheme 2. Synthesis of 10-Py-7,8-C2B9H11 and 9-Py-7,8-C2B9H11 via the reaction of nido-carborane with pyridine in the presence of HgCl2.
The asymmetrically substituted 9-pyridinium derivative of nido-carborane was also prepared via the reaction of the parent ortho-carborane with pyridine in the presence of copper acetate and water. Similar reactions with C-monosubstituted ortho-carboranes give a mixture of the corresponding isomeric pyridinium derivatives 9-Py-7-R-7,8-C2B9H10 and 11-Py-7-R-7,8-C2B9H10 (R = Me, Ph). In the case of 1-XCH2 derivatives of ortho-carborane (X = Cl, Br, OH), in addition to a mixture of the corresponding 9- and 11-pyridinium derivatives of nido-carborane, the reaction gives the pyridinium methyl derivative 7-PyCH2-7,8-C2B9H11 [55].
3. Charge-Compensated Derivatives of Nido-Carborane with Boron–Phosphorus Bond
Unlike derivatives with a boron–nitrogen bond, derivatives of nido-carboranes with a boron–phosphorus bond can be prepared using electrophilic substitution reactions. Heating the potassium salt of nido-carborane K[7,8-C2B9H12] with Ph2PCl in tetrahydrofuran at reflux leads to the P-protonated diphenylphosphonium derivative 9-Ph2PH-7,8-C2B9H11, which can be alkylated with MeI under reflux in ethanol in the presence of EtONa as a base to give the methyldiphenylphosphonium derivative 9-MePh2P-7,8-C2B9H11 (Scheme 3, Figure 3) [56][57].
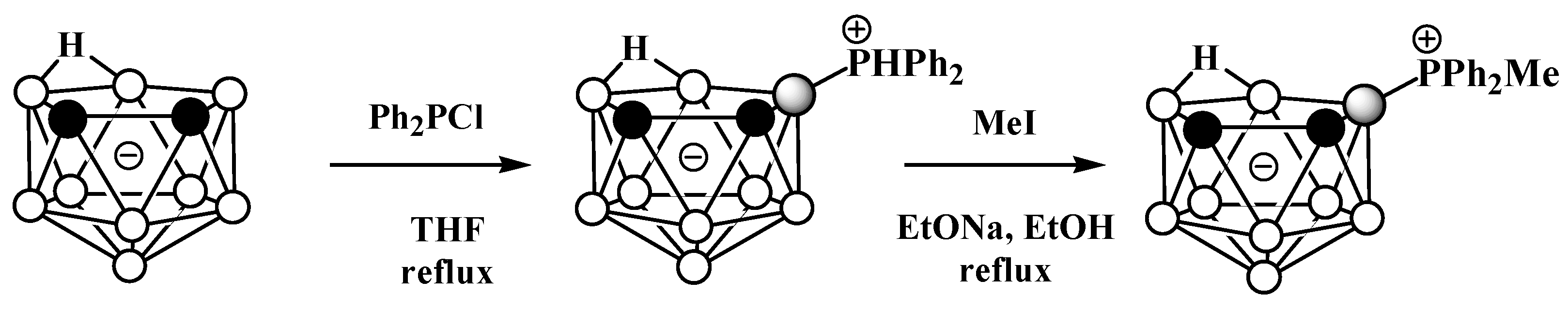

Scheme 3. The synthesis of 9-Ph2PH-7,8-C2B9H11 and its alkylation.

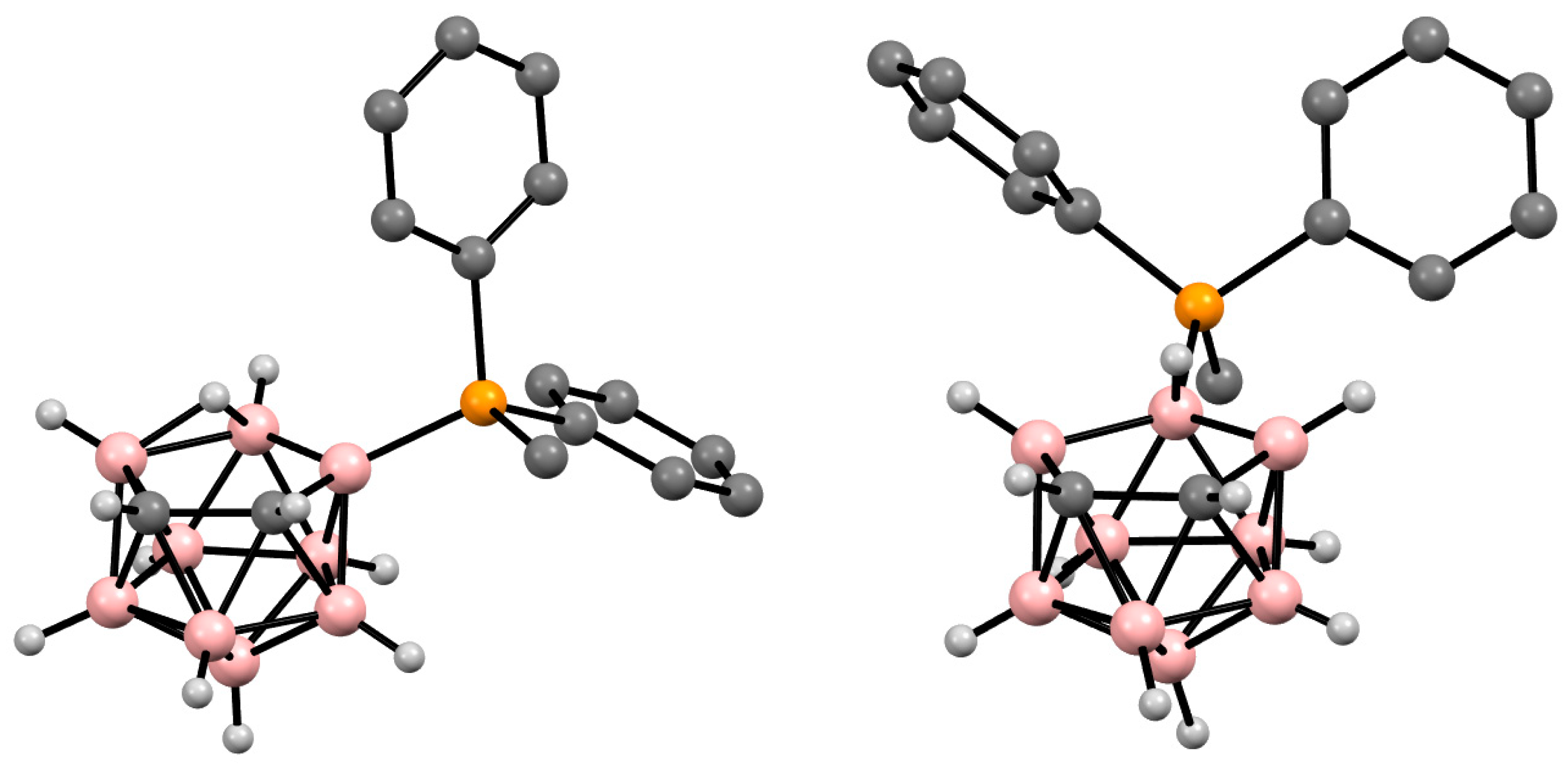
Figure 3. Crystal molecular structures of 9-MePh2P-7,8-C2B9H11 (left) and 10-MePh2P-7,8-C2B9H11 (right). Hydrogen atoms of phenyl and methyl groups are omitted for clarity.
The symmetrically substituted phosphonium derivatives 10-Ph2PH-7,8-C2B9H11 and 10-MePh2P-7,8-C2B9H11 (Figure 4) were prepared in a similar way using the disodium dicarbollide salt Na2[7,8-C2B9H11] as a starting material (Scheme 4) [57].
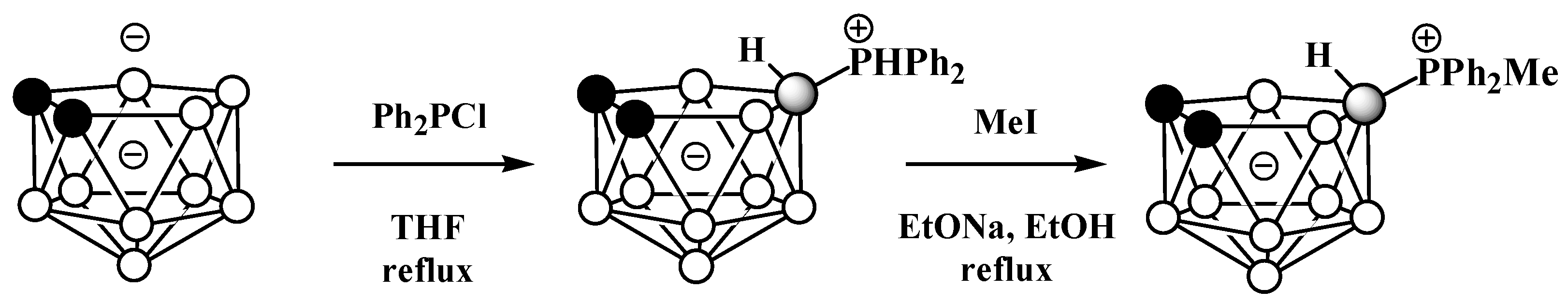

Scheme 4. The synthesis of 10-Ph2PH-7,8-C2B9H11 and its alkylation.

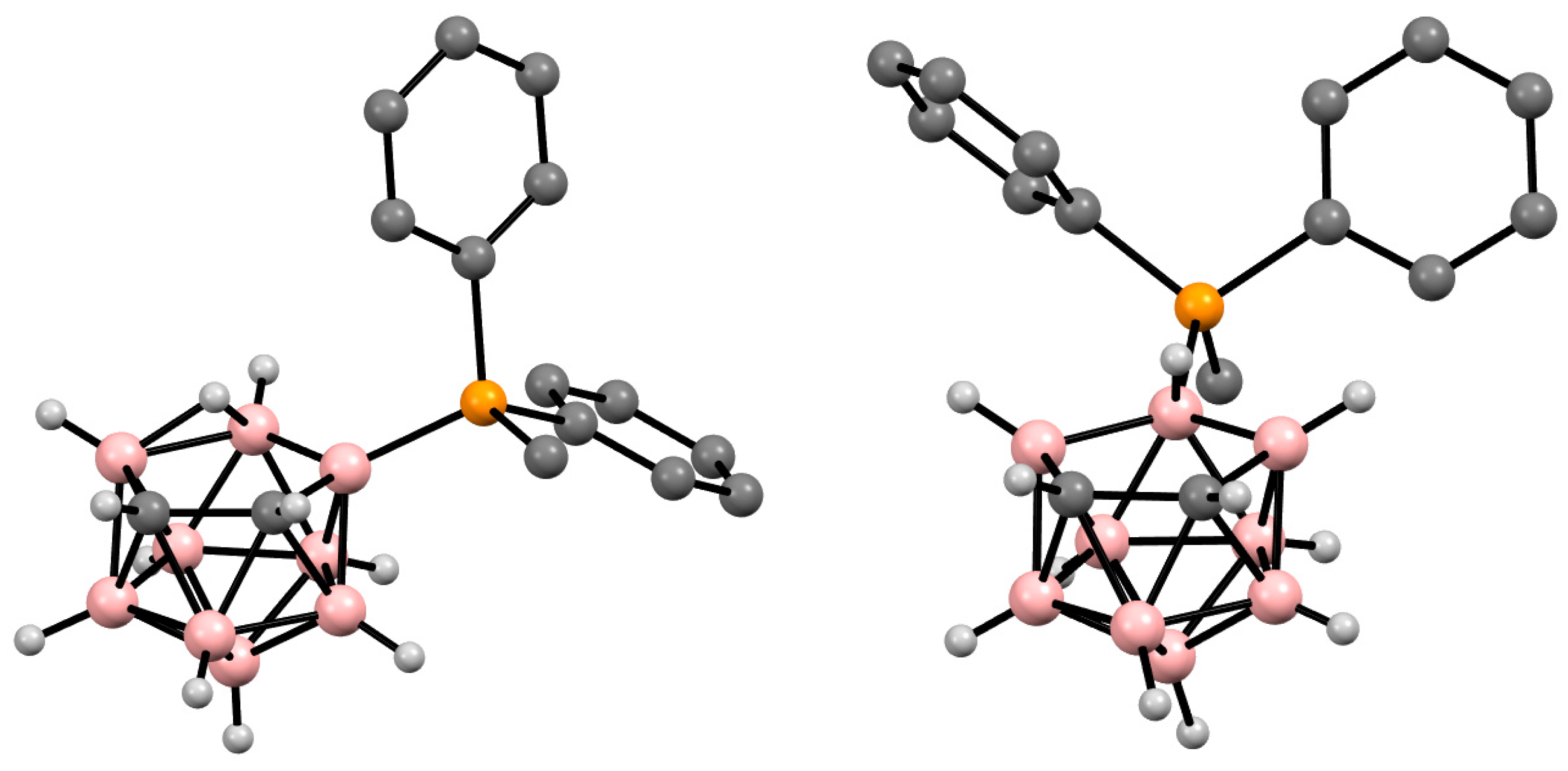
Figure 4. Crystal molecular structures of 9-MePh2P-7,8-C2B9H11 (left) and 10-MePh2P-7,8-C2B9H11 (right). Hydrogen atoms of phenyl and methyl groups are omitted for clarity.
Phosphonium derivatives of nido-carborane also can be prepared via Lewis-acid-mediated nucleophilic substitution reactions. The reaction of the potassium salt of nido-carborane K[7,8-C2B9H12] with PPh3 in the presence FeCl3 in benzene at 80 °C leads to a mixture of triphenylphosphonium 9-Ph3P-7,8-C2B9H11 and 10-Ph3P-7,8-C2B9H11, which were separated using column chromatography on silica (Scheme 5) [56][57].
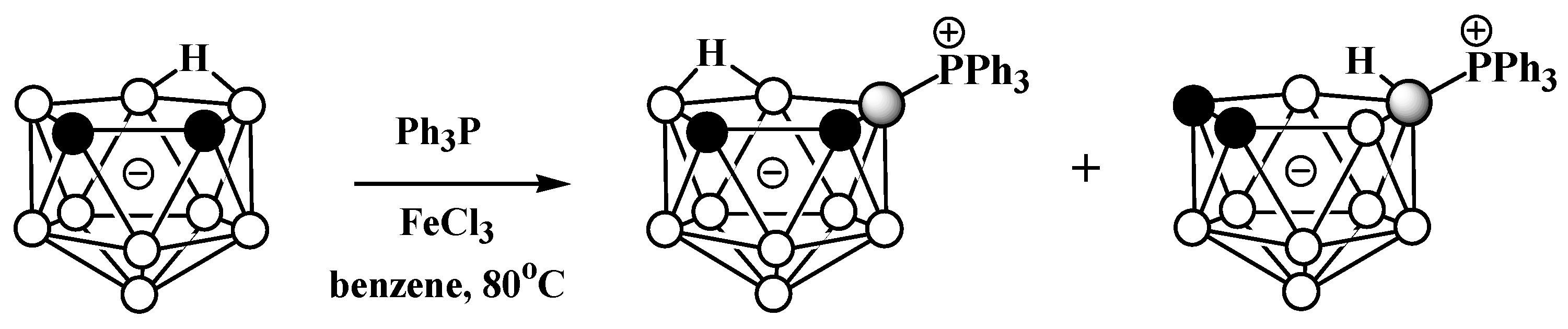

Scheme 5. Reaction of nido-carborane with triphenylphosphine in the presence of FeCl3.
The asymmetrical triphenylphosphonium derivative 9-Ph3P-7,8-C2B9H11 was also obtained via the reaction of triphenylphosphine with the dithallium dicarbollide salt Tl2[7,8-C2B9H11] in dichloromethane and in the presence of AgBr at ambient temperature (Scheme 6) [58].
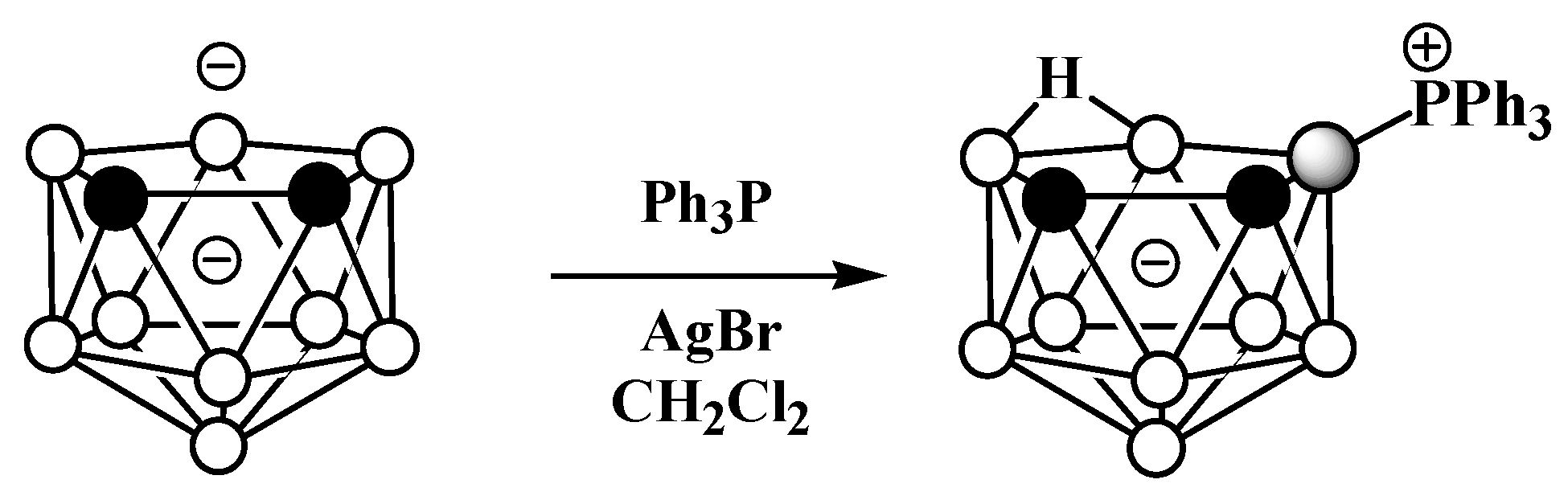

Scheme 6. Synthesis of 9-Ph3P-7,8-C2B9H11.
Similar to the pyridinium derivatives, a series of asymmetrically substituted phosphonium derivatives 9-R’R2P-7,8-Ph2-7,8-C2B9H9 was prepared via electrocatalyzed B-P oxidative couplings of 7,8-diphenyl-nido-carborane with various phosphines and phosphites (Scheme 7, Figure 5) [59].
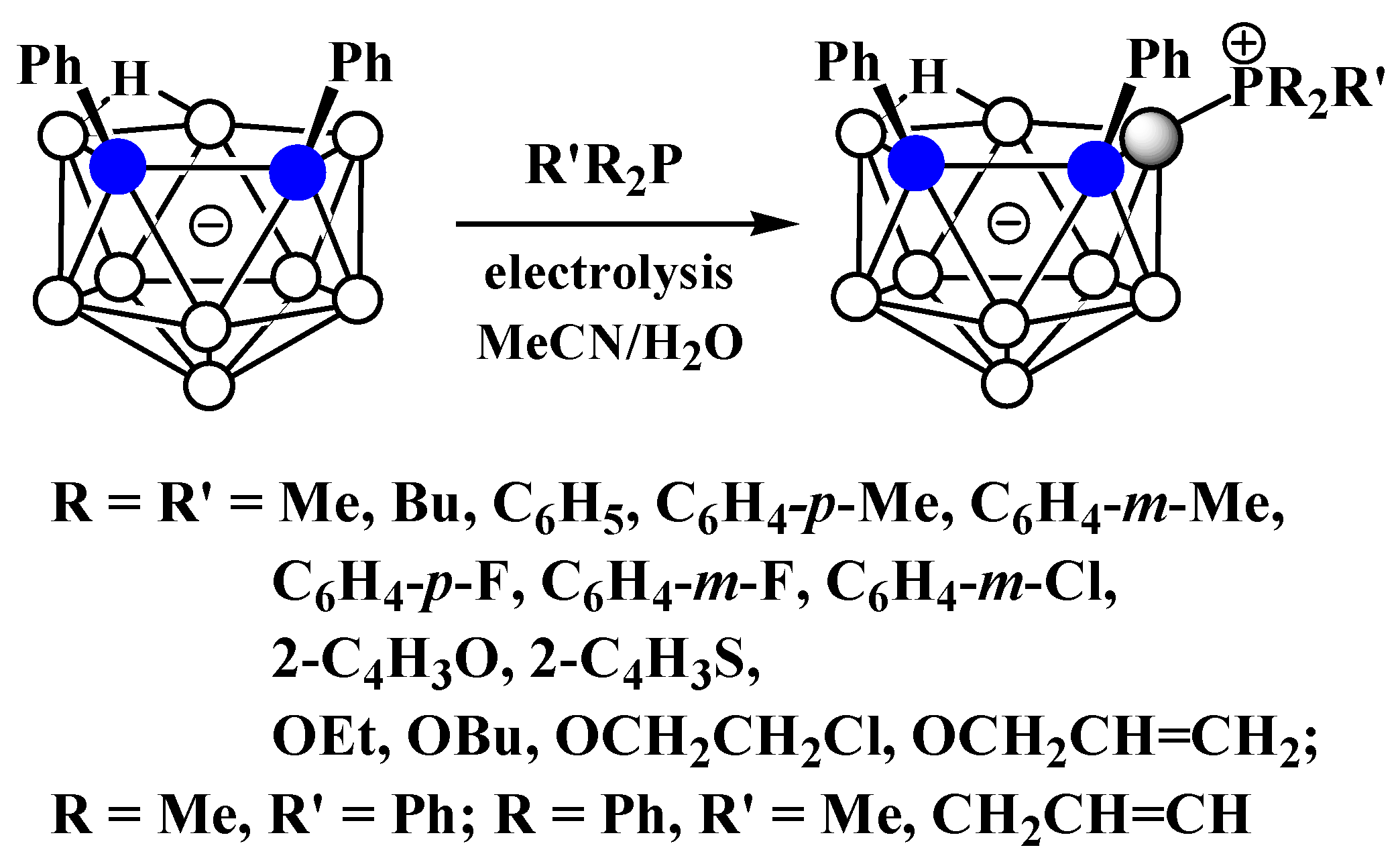
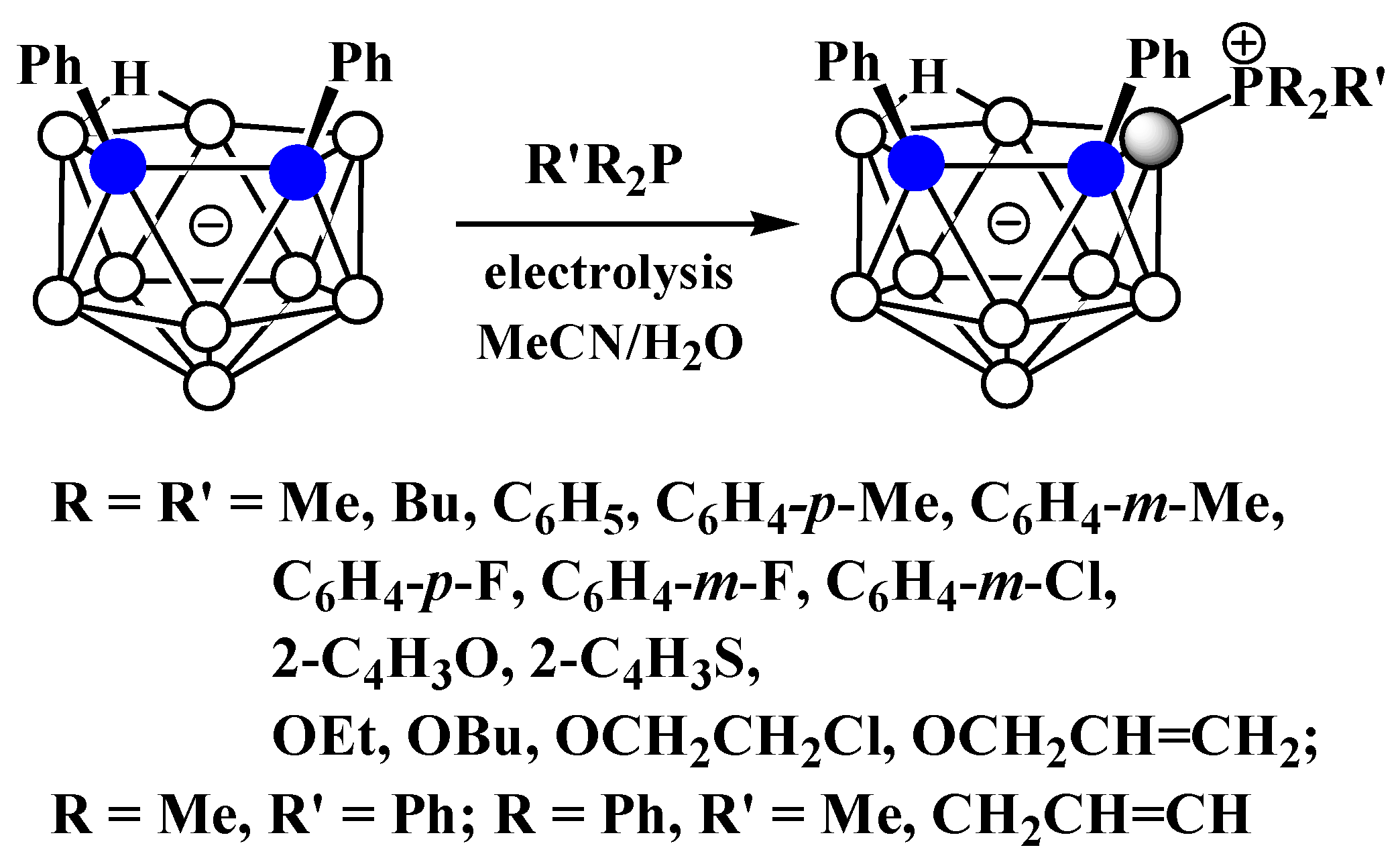
Scheme 7. The electrocatalyzed B-P oxidative coupling of triarylphosphines, diarylalkylphosphines, and trialkylphosphines with 7,8-diphenyl-nido-carborane.
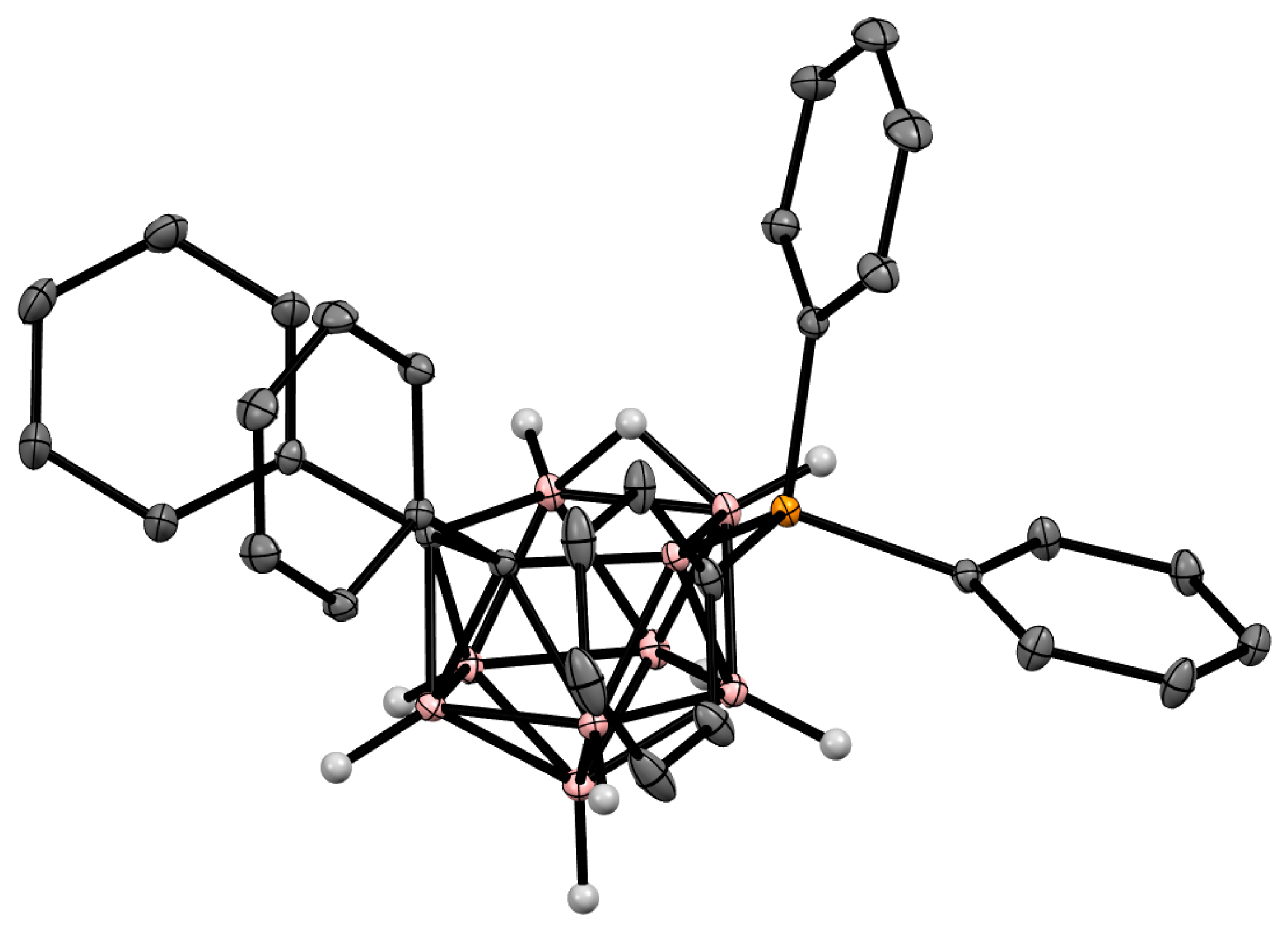

Figure 5. Crystal molecular structure of 9-Ph3P-7,8-Ph2-7,8-C2B9H9. Hydrogen atoms of phenyl rings are omitted for clarity.
4. Charge-Compensated Derivatives of Nido-Carborane with Boron–Arsenic and Boron–Antimony Bonds
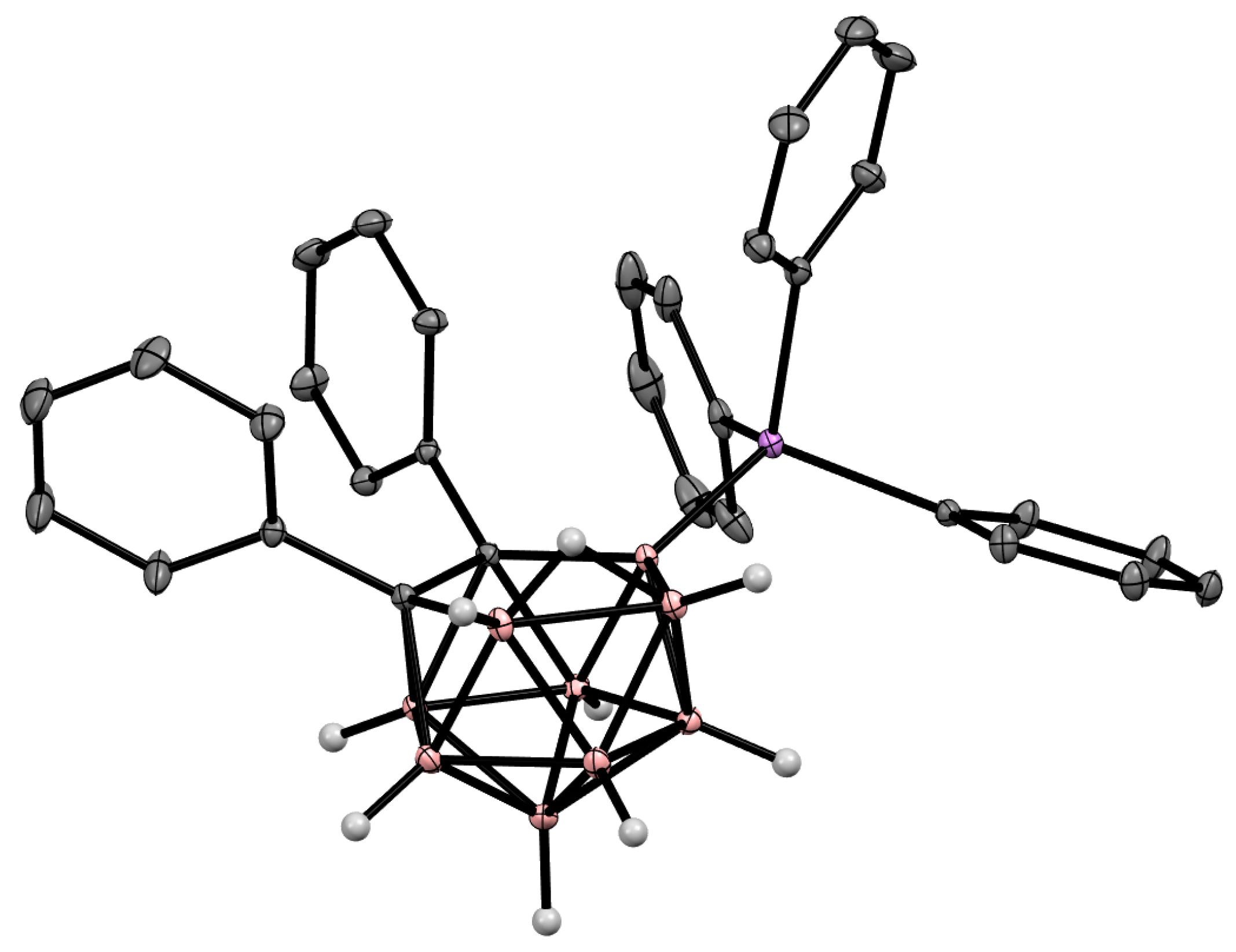
The charge-compensated derivatives of nido-carborane with boron–arsenic and boron–antimony bonds are rare and are limited to a few examples. Similar to the triphenylphosphonium derivative, the asymmetrically substituted triphenylarsonium and tetraphenylstilbonium derivatives 9-Ph3X-7,8-Ph2-7,8-C2B9H9 (X = As, Sb) were prepared via electrocatalyzed oxidative couplings of 7,8-diphenyl-nido-carborane with Ph3As and Ph3Sb, respectively (Scheme 8, Figure 6) [59].
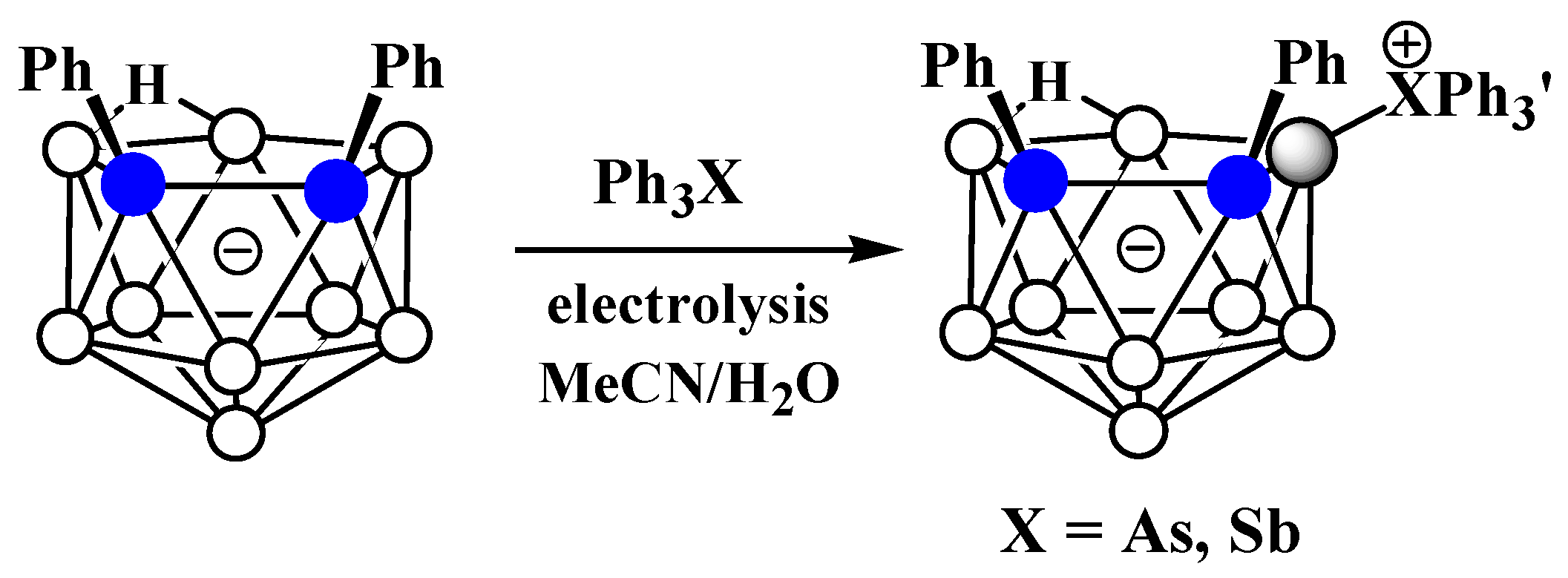
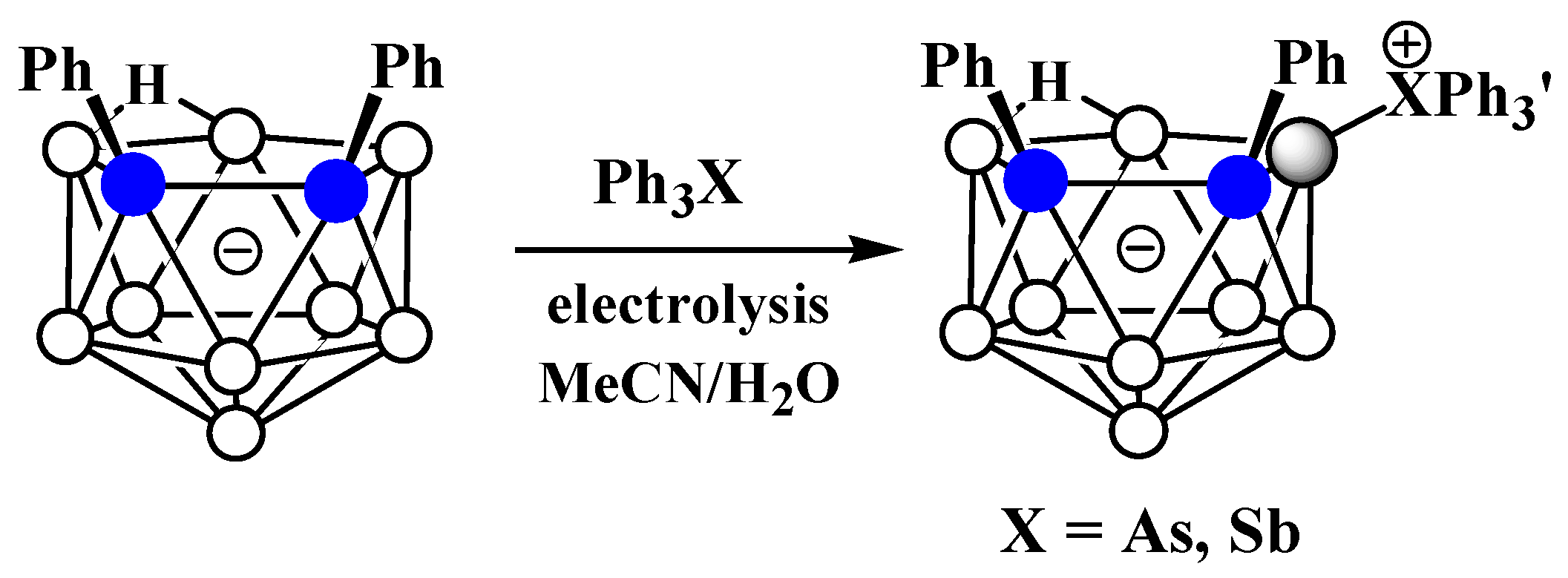
Scheme 8. Synthesis of 9-Ph3As-7,8-Ph2-7,8-C2B9H9 and 9-Ph3As-7,8-Ph2-7,8-C2B9H9.

Figure 6. Crystal molecular structure of 9-Ph3As-7,8-Ph2-7,8-C2B9H9. Hydrogen atoms of phenyl groups are omitted for clarity.
The reaction of 2-pyridyl-substituted nido-carborane [7-(2′-Py)-7,8-C2B9H11]− with triphenylarsine in the presence of catalytic amounts of PdCl2 in a mixture of toluene, water, and acetonitrile at 120°C results in the corresponding triphenylarsonium derivative 11-Ph3As-7-(2′-Py)-7,8-C2B9H10 (Scheme 9) [60].
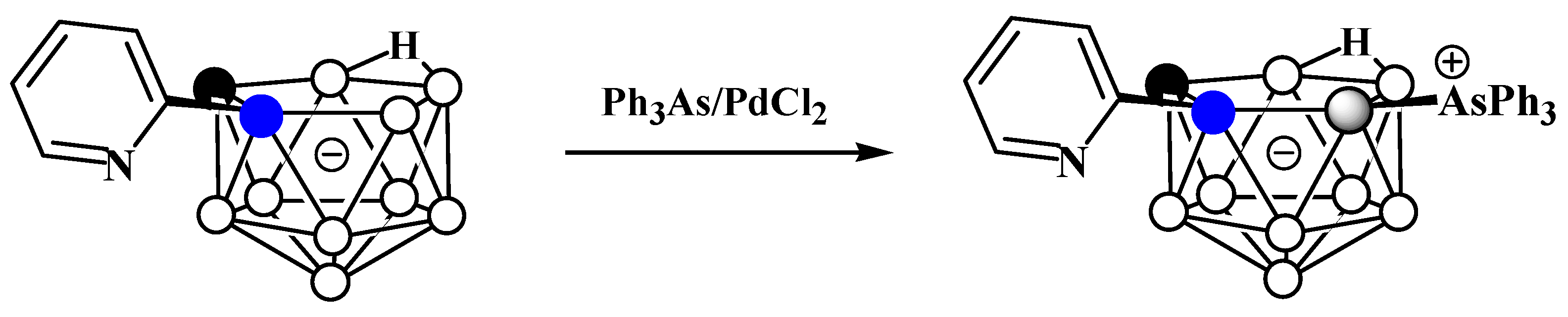
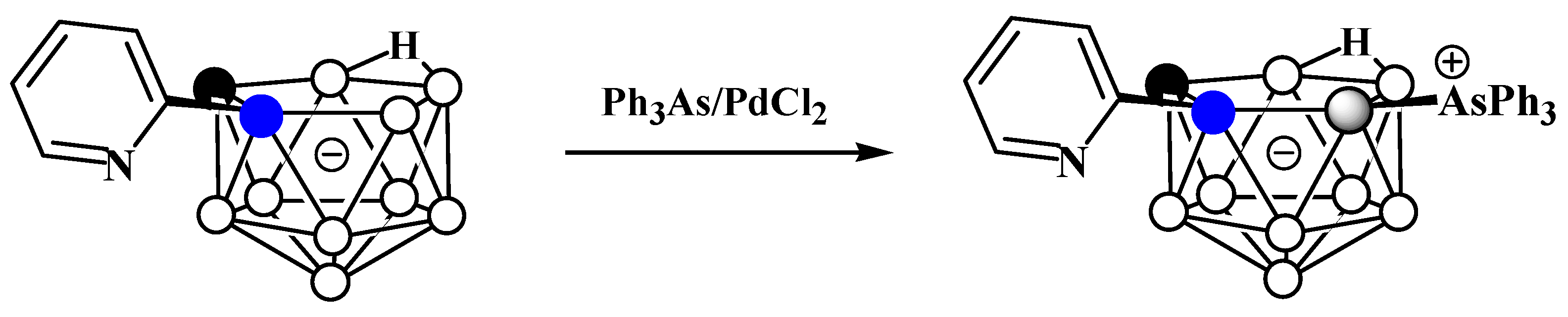
Scheme 9. Synthesis of 11-Ph3As-7-(2′-Py)-7,8-C2B9H10.
5. Charge-Compensated Derivatives of Nido-Carborane with Boron–Oxygen Bond
Alkyloxonium salts are much less stable than ammonium and phosphonium salts, and some of them are used in organic chemistry as strong alkylating agents. Nevertheless, strong electron-withdrawing of polyhedral boron hydride clusters substituted at boron atoms and, in particular, nido-carborane [61], is capable of stabilizing their oxonium derivatives [62][63]. The first example of such a derivative was obtained very soon after the discovery of nido-carborane via the reaction of the potassium salt of nido-carborane with tetrahydrofuran in the presence of FeCl3 in benzene. As a result, a mixture of two isomeric tetrahydrofuran derivatives of nido-carborane was obtained. The reaction with the C,C′-dimethyl derivative of nido-carborane proceeds in a similar way (Scheme 10) [51].
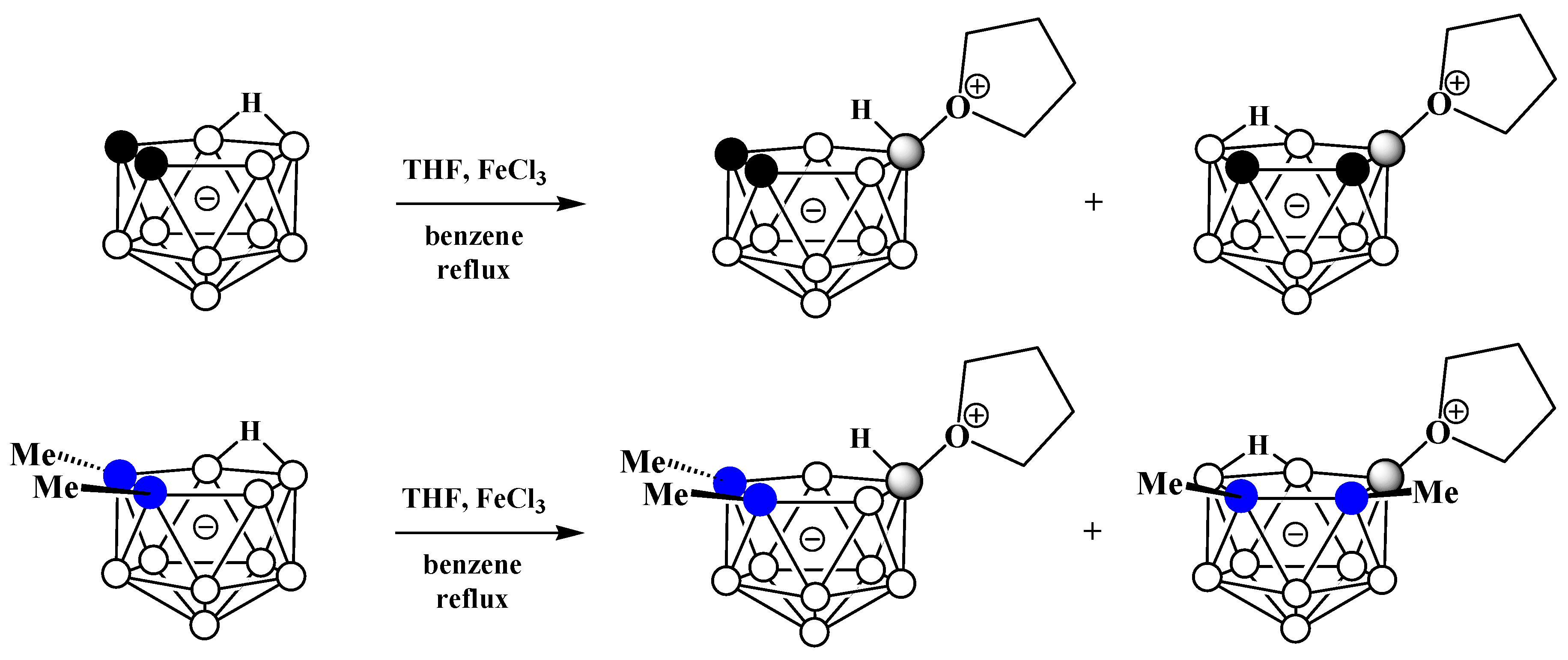

Scheme 10. Synthesis of 9-(CH2)4O-7,8-C2B9H11 and 10-(CH2)4O-7,8-C2B9H11 via the reaction of nido-carborane with FeCl3 in THF–benzene mixture.
It was later found that the replacement of FeCl3 with HgCl2 in this reaction leads to the selective formation of the symmetrically substituted tetrahydrofuran derivative 10-(CH2)4O-7,8-C2B9H11 [54][64]. The symmetrically substituted derivative was also obtained via the reaction of the tetramethylammonium salt of nido-carborane with AlCl3 in a mixture of tetrahydrofuran and acetone [65] and by the treatment of the potassium salt of nido-carborane with tetrahydrofuran in the presence of acetaldehyde or formaldehyde and hydrochloric acid in a mixture of water and toluene [66] (Scheme 11).
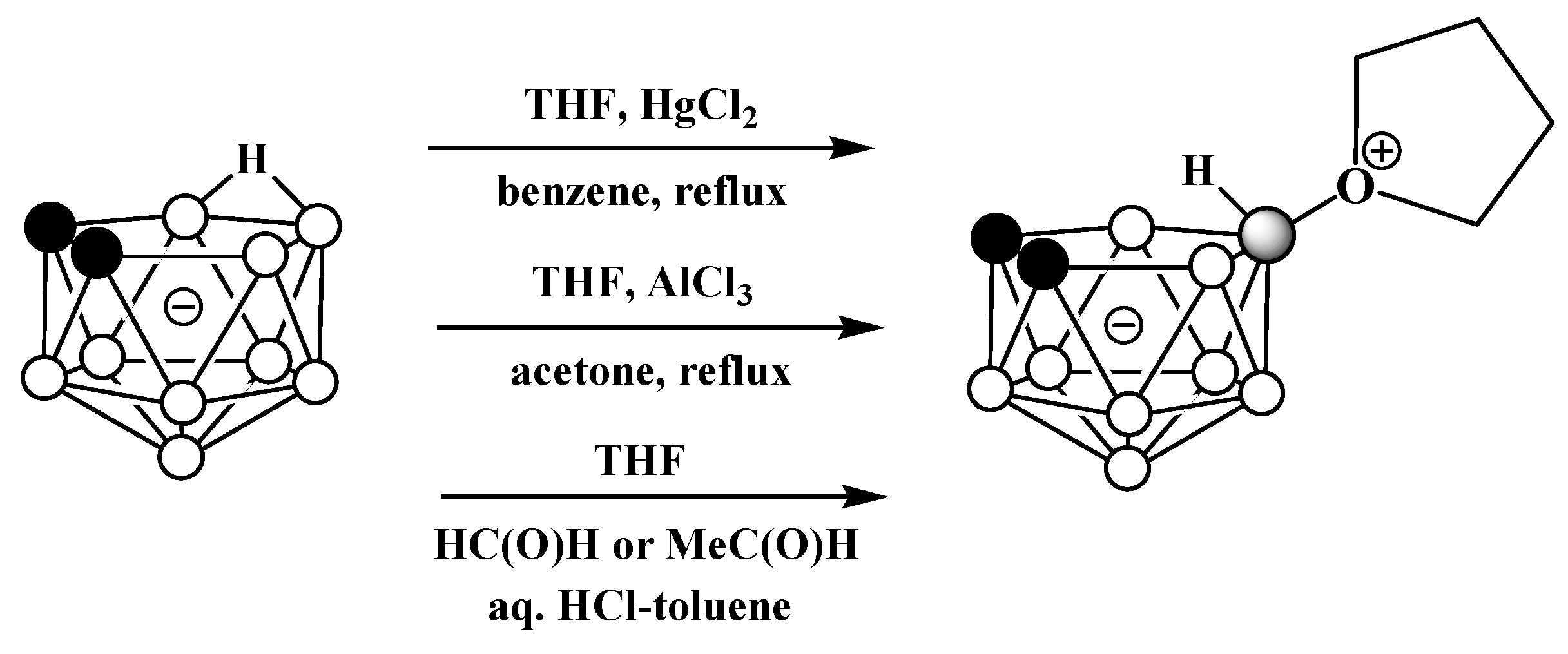

Scheme 11. Different pathways for synthesis of 10-(CH2)4O-7,8-C2B9H11.
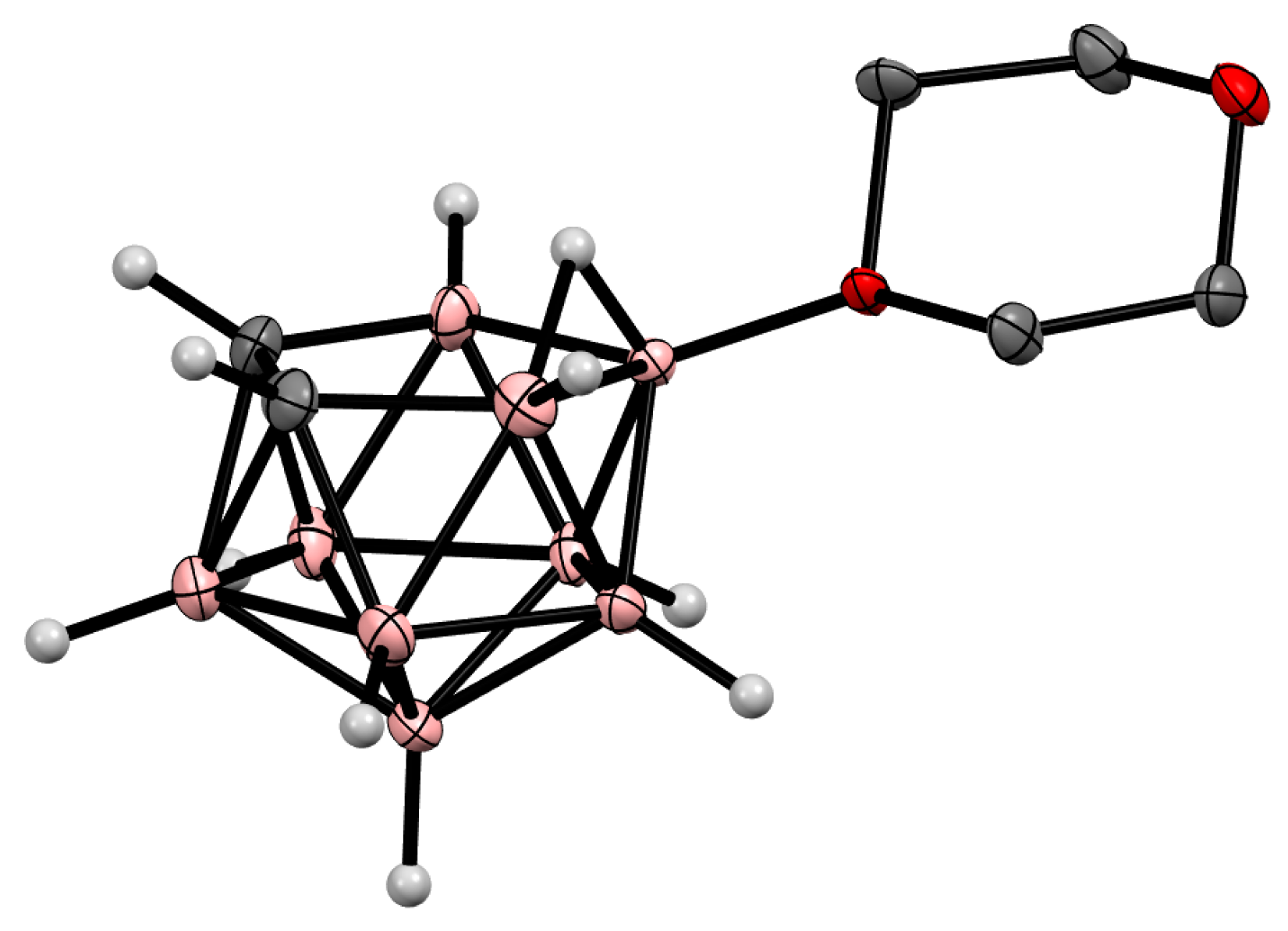
Oxonium derivatives of nido-carborane with other cyclic ethers were synthesized as well. The symmetrically substituted 1,4-dioxane derivative 10-O(CH2CH2)2O-7,8-C2B9H11 can be prepared via the reaction of the potassium salt of nido-carborane with 1,4-dioxane in the presence of HgCl2 in benzene [64] or in the presence of acetaldehyde and hydrochloric acid in a water–toluene mixture [66]. The 1,4-dioxane derivative can also be synthesized via the heating of the protonated form of nido-carborane 7,8-C2B9H13 with 1,4-dioxane [67] (Scheme 12). The molecular structure of the 1,4-dioxane derivative of nido-carborane was determined using single-crystal X-ray diffraction (Figure 7) [68].

Scheme 12. Different pathways for synthesis of 10-O(CH2CH2)2O-7,8-C2B9H11.

Figure 7. Crystal molecular structure of 10-O(CH2CH2)2O-7,8-C2B9H11. Hydrogen atoms of organic substituent are omitted for clarity.
The reaction of the potassium salt of nido-carborane with tetrahydropyran in the presence of mercury(II) chloride in benzene results in the tetrahydropyran derivative 10-(CH2)5O-7,8-C2B9H11 (Scheme 13) [69][70].

Scheme 13. Different pathways for synthesis of 10-(CH2)5O-7,8-C2B9H11.
6. Charge-Compensated Derivatives of Nido-Carborane with Boron–Sulfur Bond
Compared with the oxonium derivatives, the sulfonium derivatives of nido-carborane are represented by a wider variety of derivatives and synthetic methods for their preparation. However, the most studied of them are the dimethylsulfonium derivatives of nido-carborane, which are widely used for the synthesis of metallacarboranes [41][50][71].
It should be noted that symmetrically and asymmetrically substituted dimethylsulfonium derivatives of nido-carborane are usually obtained in different ways, which excludes the formation of mixtures of their isomers. The asymmetrically substituted 9-dimethylsulfonium derivative of nido-carborane 9-Me2S-7,8-C2B9H11 was prepared via the reaction of the parent nido-carborane with dimethylsulfoxide in the presence of sulfuric acid at 80°C [52][72][73]. The reactions of the C,C’-substituted derivatives of nido-carborane proceed in a similar way, leading to the corresponding dimethylsulfonium derivatives 9-Me2S-7,8-R2-7,8-C2B9H9 (R = Me, Ph) [52][74] (Scheme 54). These conditions are similar to those used for the synthesis of the dimethylsulfonium derivatives of the closo-decaborate [75] and closo-dodecaborate [76] anions. The C,C′-substituted derivatives 9-Me2S-7,8-Me2-7,8-C2B9H9 and 9-Me2S-7,8-μ-(1′,2′-C6H4(CH2)2)-7,8-C2B9H9 were prepared via the reactions of the corresponding nido-carboranes with dimethylsulfoxide in the presence of triflic acid in 1,2-dichloroethane [77] (Scheme 14). The structures of 9-Me2S-7,8-R2-7,8-C2B9H9 (R = H, Ph) were determined using single-crystal X-ray diffraction (Figure 8) [78][79].
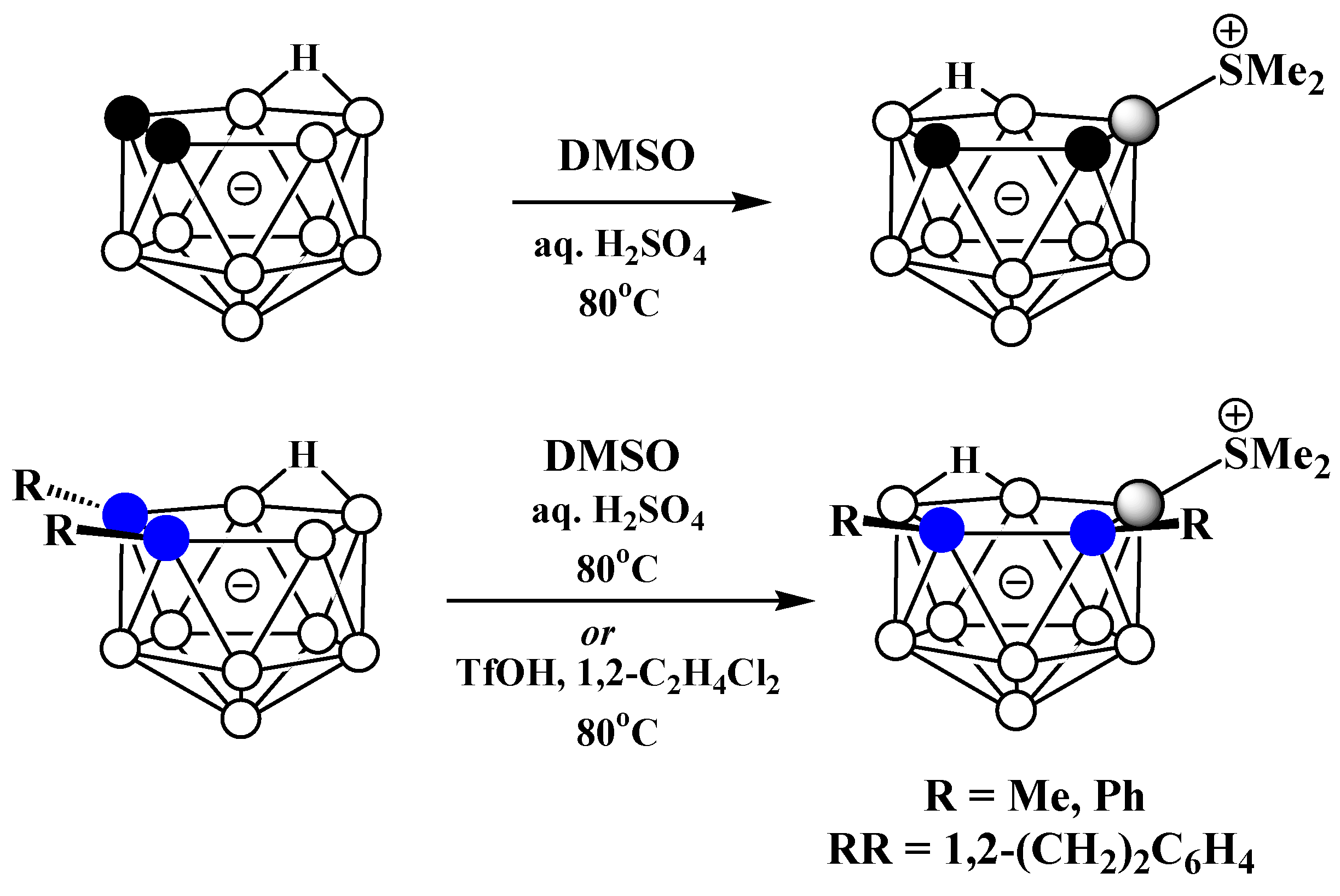

Scheme 14. Synthesis of the asymmetrically substituted dimethylsulfonium derivatives of nido-carborane 9-Me2S-7,8-R2-7,8-C2B9H9 (R = H, Me, Ph; RR = μ-1′,2′-C6H4(CH2)2).
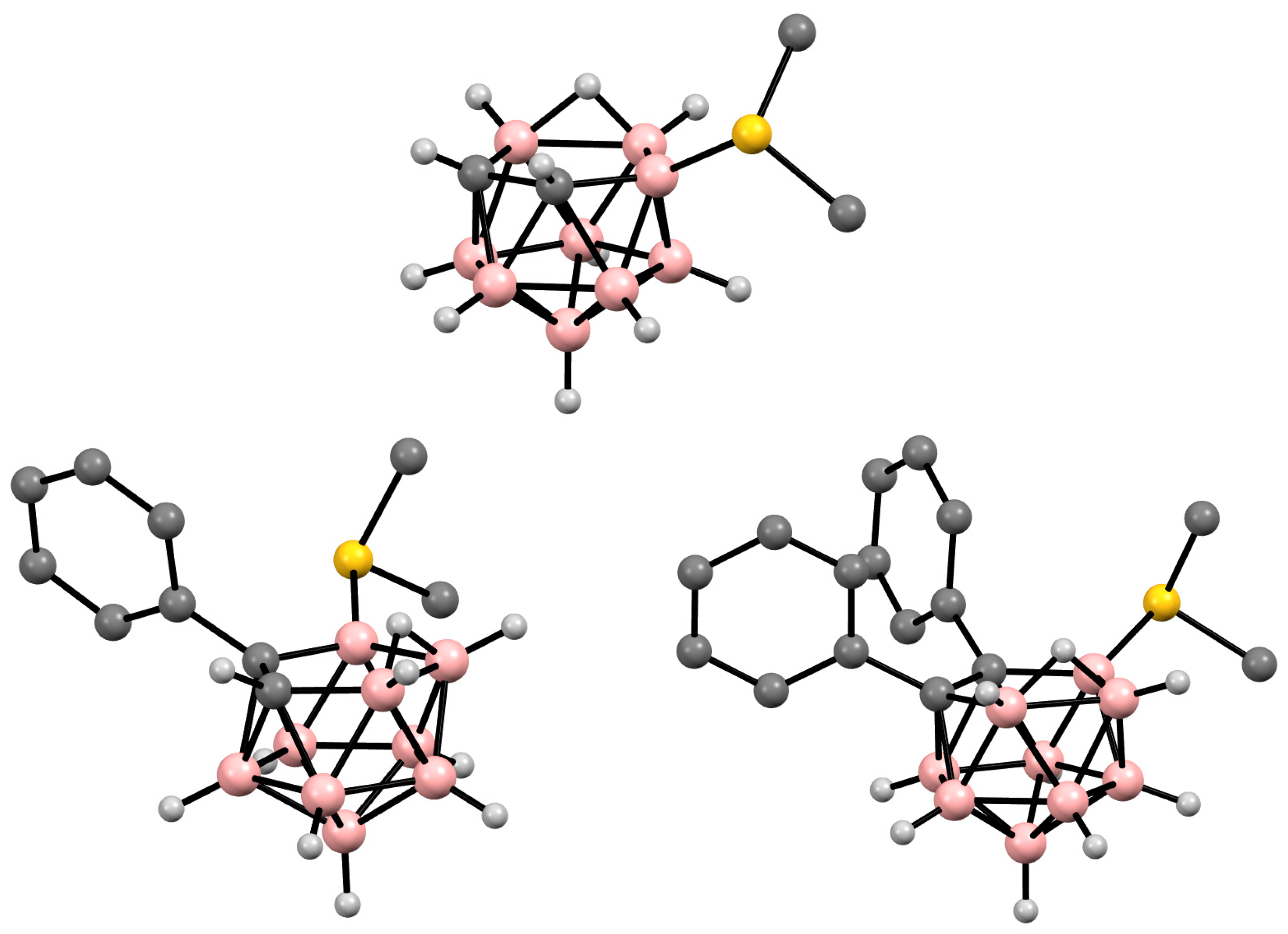

Figure 8. Crystal molecular structures of 9-Me2S-7,8-C2B9H11 (top), 11-Me2S-7-Ph-7,8-R2-7,8-C2B9H10 (bottom left), and 9-Me2S-7,8-Ph2-7,8-C2B9H9 (bottom right). Hydrogen atoms of organic substituent are omitted for clarity.
The asymmetrically substituted dimethylsulfonium derivatives 9-Me2S-7,8-Me2-7,8-C2B9H9 and 9-Me2S-7,8-µ-(CH2OCH2)-7,8-C2B9H9 were prepared via the reactions of the corresponding nido-carboranes with dimethylsulfide in the presence of Fe(NO3)3 in aqueous ethanol [49].
In the case of C-substituted nido-carboranes, such as K[7-Ph-7,8-C2B9H11], the introduction of a Me2S group results in a mixture of 9-Me2S-7-Ph-7,8-C2B9H10 and 11-Me2S-7-Ph-7,8-C2B9H10 isomers, which can be separated using column chromatography [73]. The molecular structure of 11-Me2S-7-Ph-7,8-C2B9H10 was determined using single-crystal X-ray diffraction (Figure 8) [74].
7. Charge-Compensated Derivatives of Nido-Carborane with Boron–Selenium and Boron–Tellurium Bonds
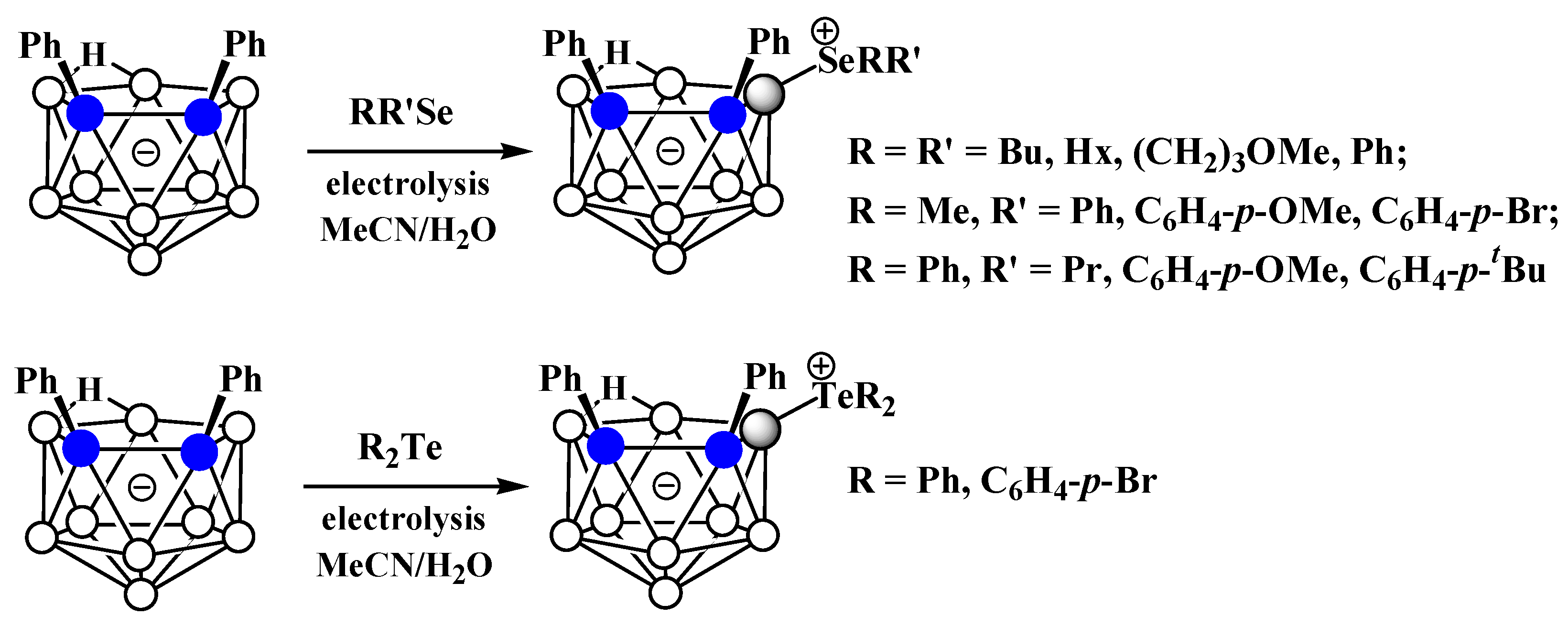
The charge-compensated derivatives of nido-carborane with boron–selenium and boron–tellurium bonds are rather rare. Similar to the dialkyl- and diarylsulfonium derivatives, a series of asymmetrically substituted triakyl(aryl)selenium and triaryltellurium derivatives 9-RR’X-7,8-Ph2-7,8-C2B9H9 (X = Se, Te) were prepared via electrocatalyzed oxidative couplings of 7,8-diphenyl-nido-carborane with RR’Se and R2Te, respectively (Scheme 15, Figure 9) [59].
Scheme 15. Synthesis of 9-dialkyl(aryl)selenium and 9-diaryltellurium derivatives of nido-carborane 9-RR’X-7,8-Ph2-7,8-C2B9H9.

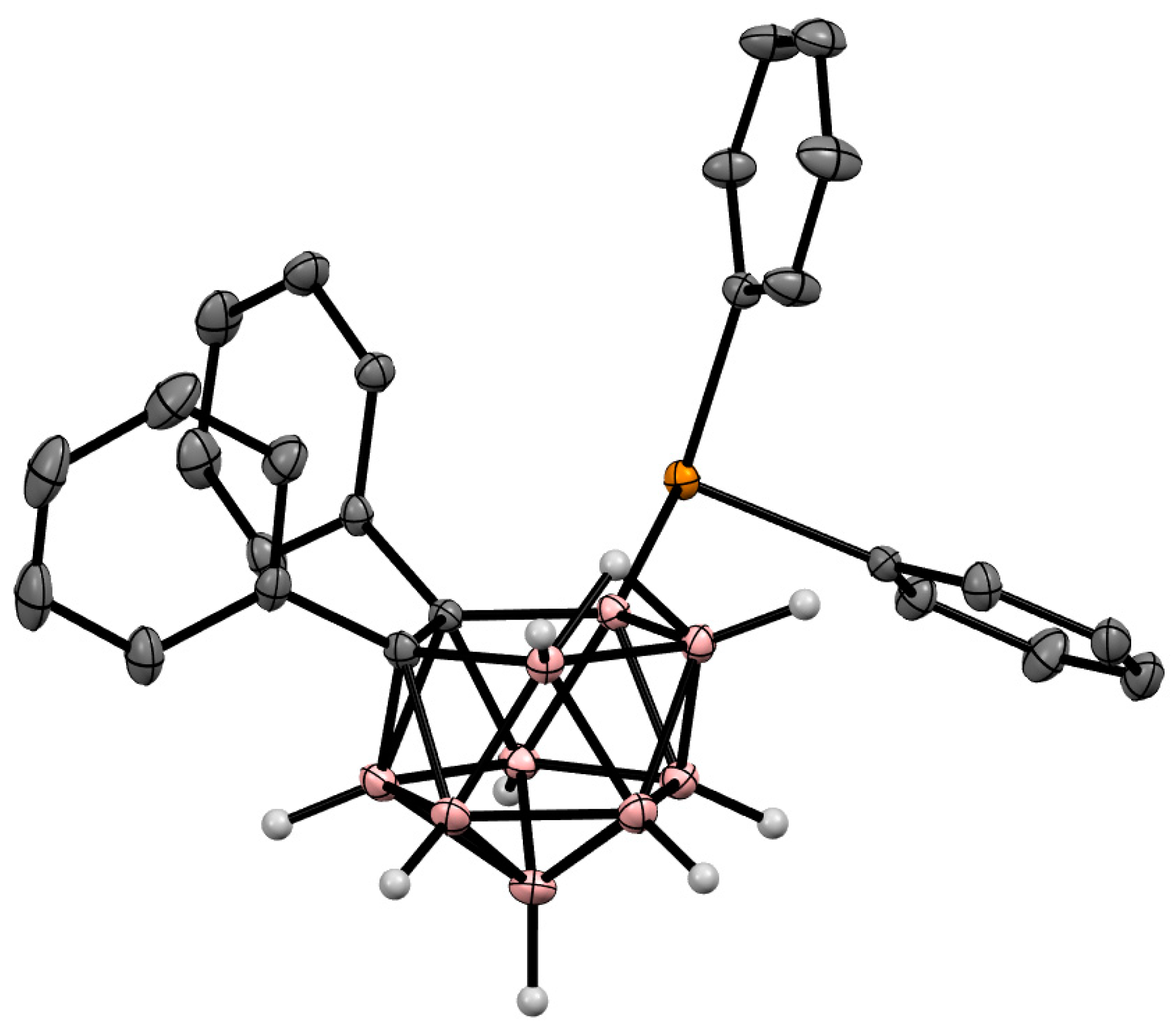
Figure 9. Crystal molecular structure of 9-Ph2Te-7,8-Ph2-7,8-C2B9H9. Hydrogen atoms of phenyl groups are omitted for clarity.
8. Some Other Charge-Compensated Derivatives of Nido-Carborane
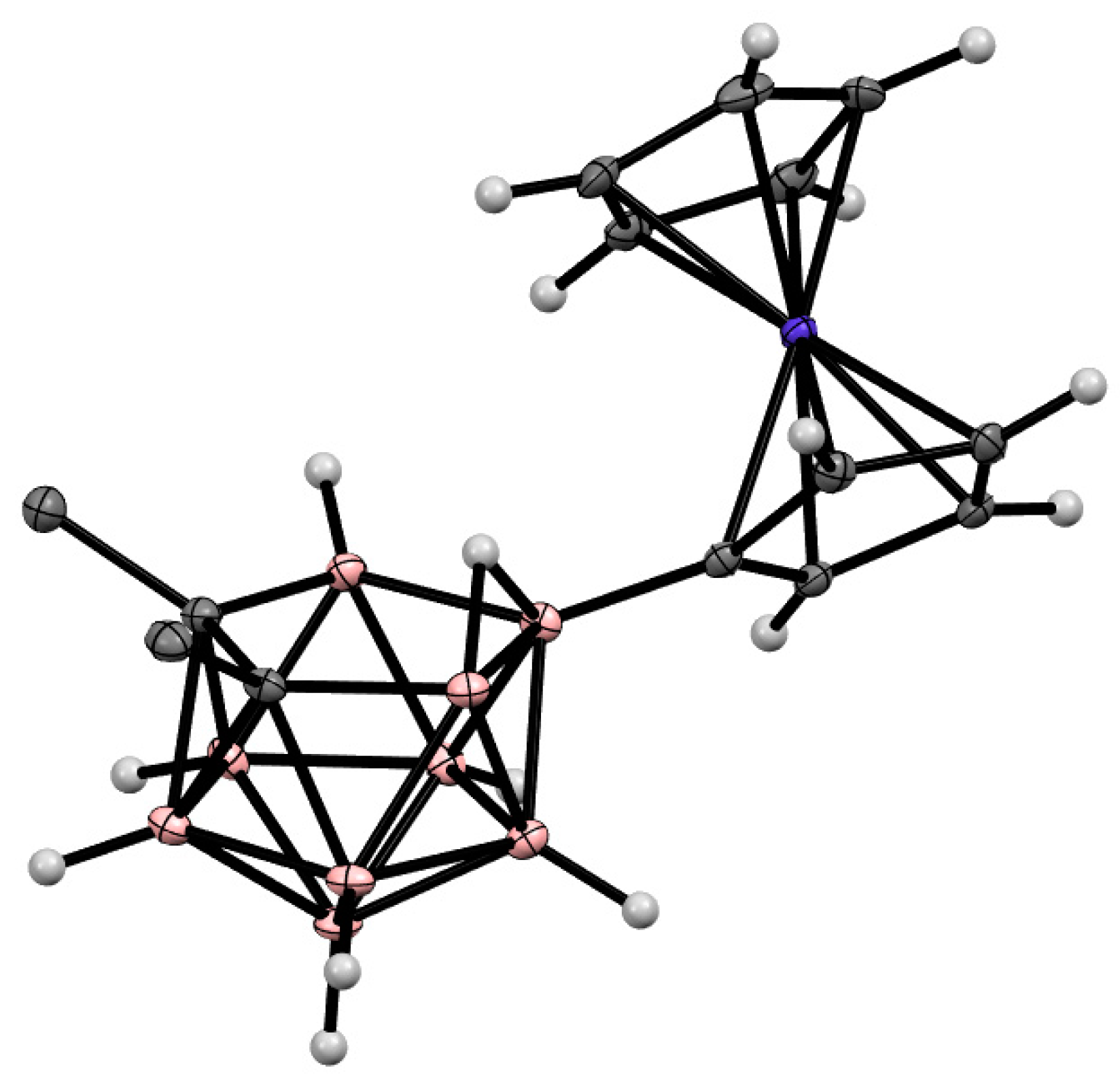
The asymmetrically substituted 9-carbonyl derivative of nido-carborane 9-O≡C-7,8-C2B9H11 and the 3,3,8-(CO)3-3,1,2-CoC2B9H10 cobaltacarborane based on its symmetrically substituted analog as a ligand were isolated as minor products of the reaction of the parent nido-carborane with [Co2(CO)8] [80]. The symmetrically substituted cobaltacenium derivative of nido-carborane 10-{CpCo(C5H4)}-7,8-Me2-7,8-C2B9H9 (Figure 10) was prepared along with the 3-Cp-1,2-Me2-3,1,2-CoC2B9H9 cobaltacarborane in the reaction of the dithallium dicarbollide salt Tl2[7,8-Me2-7,8-C2B9H9] with CpCo(CO)I2 in acetonitrile [81].
Figure 10. Crystal molecular structure of 10-{CpCo(C5H4)}-7,8-Me2-7,8-C2B9H9. Hydrogen atoms of methyl groups are omitted for clarity.
References
- McCleverty, J.A. Highlights in inorganic chemistry over the last 100 years. Annu. Rep. Prog. Chem. Sect. A 2004, 100, 3–13.
- Heying, T.L.; Ager, J.W.; Clark, S.L.; Mangold, D.J.; Goldstein, H.L.; Hillman, M.; Polak, R.J.; Szymanski, J.W. A new series of organoboranes. I. Carboranes from the reaction of decaborane with acetylenic compounds. Inorg. Chem. 1963, 2, 1088–1092.
- Fein, M.M.; Grafstein, D.; Paustian, J.E.; Bobinski, J.; Lichstein, B.M.; Mayes, N.; Schwartz, N.; Cohen MSCarboranes, I.I. The preparation of 1- and 1,2-substituted carboranes. Inorg. Chem. 1963, 2, 1115–1119.
- Zakharkin, L.I.; Stanko, V.I.; Brattsev, V.A.; Chapovskii, Y.A.; Okhlobystin, Y.O. Synthesis of a new class of organoboron compounds, B10C2H12 (barene) and its derivatives. Bull. Acad. Sci. USSR Div. Chem. Sci. 1963, 12, 2074.
- Zakharkin, L.I.; Stanko, V.I.; Brattstev, V.A.; Chapovskii, Y.A.; Struchkov, Y.T. The structure of B10C2H12 (barene) and its derivatives. Bull. Acad. Sci. USSR Div. Chem. Sci. 1963, 12, 1911.
- Bregadze, V.I. Fifty years of carborane chemistry: The history of discovery and the first results. Russ. Chem. Bull. 2014, 63, 1021–1026.
- Wiesboeck, R.A.; Hawthorne, M.F. Dicarbaundecaborane(13) and derivatives. J. Am. Chem. Soc. 1964, 86, 1642–1643.
- Hawthorne, M.F.; Young, D.C.; Garrett, P.M.; Owen, D.A.; Schwerin, S.G.; Tebbe, F.N.; Wegner, P.A. Preparation and characterization of the (3)-1,2- and (3)-1,7-dicarbadodecahydroundecaborate(-1) ions. J. Am. Chem. Soc. 1968, 90, 862–868.
- Hawthorne, M.F.; Young, D.C.; Andrews, T.D.; Howe, D.V.; Pilling, R.L.; Pitts, A.D.; Reintjer, M.; Warren, L.F.; Wegner, P.A. π-Dicarbollyl derivatives of the transition metals. Metallocene analogs. J. Am. Chem. Soc. 1968, 90, 879–896.
- Grimes, R.N. Transition metal metallacarbaboranes. In Comprehensive Organometallic Chemistry II; Elsevier: Oxford, UK, 1995; Volume 1, pp. 373–430.
- Sivaev, I.B.; Bregadze, V.I. Chemistry of cobalt bis(dicarbollides). A review. Collect. Czech. Chem. Commun. 1999, 64, 783–805.
- Grimes, R.N. Metallacarboranes in the new millennium. Coord. Chem. Rev. 2000, 200, 773–811.
- Sivaev, I.B.; Bregadze, V.I. Chemistry of nickel and iron bis(dicarbollides). A review. J. Organomet. Chem. 2002, 614–615, 27–36.
- Hosmane, N.S.; Maguire, J.A. Metallacarboranes of d- and f-block metals. In Comprehensive Organometallic Chemistry III; Elsevier: Oxford, UK, 2007; Volume 3, pp. 175–264.
- Grimes, R.N. Metallacarboranes of the transition and lanthanide elements. In Carboranes, 3rd ed.; Academic Press: London, UK, 2016; pp. 711–903.
- Dash, B.P.; Satapathy, R.; Swain, B.R.; Mahanta, C.S.; Jena, B.B.; Hosmane, N.S. Cobalt bis(dicarbollide) anion and its derivatives. J. Organomet. Chem. 2017, 849, 170–194.
- Kar, S.; Pradhan, A.N.; Ghosh, S. Polyhedral metallaboranes and metallacarboranes. In Comprehensive Organometallic Chemistry IV; Elsevier: Oxford, UK, 2022; Volume 9, pp. 263–369.
- Pak, R.H.; Primus, F.J.; Rickard-Dickson, K.J.; Ng, L.L.; Kane, R.R.; Hawthorne, M.F. Preparation and properties of nido-carborane-specific monoclonal antibodies for potential use in boron neutron capture therapy for cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 6986–6990.
- Hogenkamp, H.P.C.; Collins, D.A.; Live, D.; Benson, L.M.; Naylor, S. Synthesis and characterization of nido-carborane-cobalamin conjugates. Nucl. Med. Biol. 2000, 27, 89–92.
- Tolmachev, V.; Bruskin, A.; Sjöberg, S.; Carlsson, J.; Lundqvist, H. Preparation, radioiodination and in vitro evaluation of a nido-carborane-dextran conjugate, a potential residualizing label for tumor targeting proteins and peptides. J. Radioanal. Nucl. Chem. 2004, 261, 107–112.
- Winberg, K.J.; Persson, M.; Malmström, P.-U.; Sjöberg, S.; Tolmachev, V. Radiobromination of anti-HER2/neu/ErbB-2 monoclonal antibody using the p-isothiocyanatobenzene derivative of the undecahydro-bromo-7,8-dicarba-nido-undecaborate(1-) ion. Nucl. Med. Biol. 2004, 31, 425–433.
- Wilbur, D.S.; Chyan, M.-K.; Hamlin, D.K.; Kegley, B.B.; Risler, R.; Pathare, P.M.; Quinn, J.; Vessella, R.L.; Foulon, C.; Zalutsky, M.; et al. Reagents for astatination of biomolecules: Comparison of the in vivo distribution and stability of some radioiodinated/astatinated benzamidyl and nido-carboranyl compounds. Bioconjug. Chem. 2004, 15, 203–223.
- Wilkinson, S.M.; Gunosewoyo, H.; Barron, M.L.; Boucher, A.; McDonnell, M.; Turner, P.; Morrison, D.E.; Bennett, M.R.; McGregor, I.S.; Rendina, L.M.; et al. The first CNS-active carborane: A novel P2X7 receptor antagonist with antidepressant activity. ACS Chem. Neurosci. 2014, 5, 335–339.
- El-Zaria, M.E.; Genady, A.R.; Janzen, N.; Petlura, C.I.; Beckford Vera, D.R.; Valliant, J.F. Preparation and evaluation of carborane-derived inhibitors of prostate specific membrane antigen (PSMA). Dalton Trans. 2014, 43, 4950–4961.
- Neumann, W.; Xu, S.; Sárosi, M.B.; Scholz, M.S.; Crews, B.C.; Ghebreselasie, K.; Banerjee, S.; Marnett, L.J.; Hey-Hawkins, E. nido-Dicarbaborate induces potent and selective inhibition of cyclooxygenase-2. ChemMedChem 2016, 11, 175–178.
- Różycka, D.; Korycka-Machała, M.; Żaczek, A.; Dziadek, J.; Gurda, D.; Orlicka-Płocka, M.; Wyszko, E.; Biniek-Antosiak, K.; Rypniewski, W.; Olejniczak, A.B. Novel isoniazid-carborane hybrids active in vitro against Mycobacterium tuberculosis. Pharmaceuticals 2020, 13, 465.
- Useini, L.; Mojić, M.; Laube, M.; Lönnecke, P.; Dahme, J.; Sárosi, M.B.; Mijatović, S.; Maksimović-Ivanić, D.; Pietzsch, J.; Hey-Hawkins, E. Carboranyl analogues of mefenamic acid and their biological evaluation. ACS Omega 2022, 7, 24282–24291.
- Crespo, O.; Díez-Gil, C.; Gimeno, M.C.; Jones, P.G.; Laguna, A.; Ospino, I.; Tapias, J.; Villacampa, M.D.; Visbal, R. Influence of the group 11 metal on the emissive properties of complexes . Dalton Trans. 2013, 42, 8298–8306.
- Wang, B.; Shelar, D.P.; Han, X.-Z.; Li, T.-T.; Guan, X.; Lu, W.; Liu, K.; Chen, Y.; Fu, W.-F.; Che, C.-M. Long-lived excited states of zwitterionic copper(I) complexes for photoinduced cross-dehydrogenative coupling reactions. Chem. Eur. J. 2015, 21, 1184–1190.
- Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Electron-donating abilities and luminescence properties of tolane-substituted nido-carboranes. N. J. Chem. 2017, 41, 10550–10554.
- Shafikov, M.Z.; Suleymanova, A.F.; Czerwieniec, R.; Yersin, H. Thermally activated delayed fluorescence from Ag(I) complexes: A route to 100% quantum yield at unprecedentedly short decay time. Inorg. Chem. 2017, 56, 13274–13285.
- Shafikov, M.Z.; Suleymanova, A.F.; Czerwieniec, R.; Yersin, H. Design strategy for Ag(I)-based thermally activated delayed fluorescence reaching an efficiency breakthrough. Chem. Mater. 2017, 29, 1708–1715.
- Nghia, N.V.; Jana, S.; Sujith, S.; Ryu, J.Y.; Lee, J.; Lee, S.U.; Lee, M.H. nido-Carboranes: Donors for thermally activated delayed fluorescence. Angew. Chem. Int. Ed. 2018, 57, 12483–12488.
- Nishino, K.; Hashimoto, K.; Tanaka, K.; Morisaki, Y.; Chujo, Y. Comparison of luminescent properties of helicene-like bibenzothiophenes with o-carborane and 5,6-dicarba-nido-decaborane. Sci. Chem. Chin. 2018, 61, 940–946.
- Shafikov, M.Z.; Suleymanova, A.F.; Schinabeck, A.; Yersin, H. Dinuclear Ag(I) complex designed for highly efficient thermally activated delayed fluorescence. J. Phys. Chem. Lett. 2018, 9, 702–709.
- Sujith, S.; Nam, E.B.; Lee, J.; Lee, S.U.; Lee, M.H. Enhancing the thermally activated delayed fluorescence of nido-carborane-appended triarylboranes by steric modification of the phenylene linker. Inorg. Chem. Front. 2020, 7, 3456–3464.
- Kim, M.; Im, S.; Ryu, C.H.; Lee, S.H.; Hong, J.H.; Lee, K.M. Impact of deboronation on the electronic characteristics of closo-o-carborane: Intriguing photophysical changes in triazole-appended carboranyl luminophores. Dalton Trans. 2021, 50, 3207–3215.
- Alconchel, A.; Crespo, O.; García-Orduña, P.; Gimeno, M.C. closo- or nido-Carborane diphosphane as responsible for strong thermochromism or time activated delayed fluorescence (TADF) in 0/+. Inorg. Chem. 2021, 60, 18521–18528.
- Uemura, K.; Tanaka, K.; Chujo, Y. Conformation-dependent electron donation of nido-carborane substituents and its influence on phosphorescence of tris(2,2′-bipyridyl)ruthenium(II) complex. Crystals 2022, 12, 688.
- Zhu, M.; Zhou, Q.; Cheng, H.; Sha, Y.; Bregadze, V.I.; Yan, H.; Sun, Z.; Li, X. Boron-cluster embedded necklace-shaped nanohoops. Angew. Chem. Int. Ed. 2022, 61, e202213470.
- Timofeev, S.V.; Sivaev, I.B.; Prikaznova, E.A.; Bregadze, V.I. Transition metal complexes with charge-compensated dicarbollide ligands. J. Organomet. Chem. 2014, 751, 221–250.
- Vinogradov, M.M.; Nelyubina, Y.V.; Loginov, D.A.; Kudinov, A.R. Demethylation of the SMe2 substituent in cationic metallacarboranes. Halide anion influence. J. Organomet. Chem. 2015, 798, 257–262.
- Vinogradov, M.M.; Nelyubina, Y.V.; Ikonnikov, N.S.; Strelkova, T.V.; Kudinov, A.R. First metallacarborane ethene complex and its reaction with iodine. J. Organomet. Chem. 2016, 805, 54–58.
- Vinogradov, M.M.; Nelyubina, Y.V.; Pavlov, A.P.; Novikov, V.V.; Shvydkiy, N.V.; Kudinov, A.R. Polyhedral rearrangements in the complexes of rhodium and iridium with isomeric carborane anions − (X = 9 and 10). Organometallics 2017, 36, 791–800.
- Timofeev, S.V.; Zhidkova, O.B.; Sivaev, I.B.; Starikova, Z.A.; Suponitsky, K.Y.; Yan, H.; Bregadze, V.I. Synthesis of rhodacarboranes containing σ- and π-carboranyl ligands in one molecule. J. Organomet. Chem. 2018, 867, 342–346.
- Vinogradov, M.M.; Nelyubina, Y.V.; Ikonnikov, N.S. Different reactivity of cyclooctadiene complexes 3,3-(cod)-8-SMe2-closo-3,1,2-RhC2B9H10 and 1,8-Me2-2,2-(cod)-11-SMe2-2,1,8-closo-RhC2B9H8 toward iodine. J. Organomet. Chem. 2018, 867, 224–227.
- Vinogradov, M.M.; Loginov, D.A. Rhoda- and iridacarborane halide complexes: Synthesis, structure and application in homogeneous catalysis. J. Organomet. Chem. 2020, 910, 121135.
- Timofeev, S.V.; Zhidkova, O.B.; Suponitsky, K.Y.; Anisimov, A.A.; Sivaev, I.B.; Yan, H.; Bregadze, V.I. Rhodacarboranes containing σ- and π-carborane ligands. New aspects. Inorg. Chim. Acta 2021, 518, 120243.
- Vinogradov, M.M.; Nesterov, I.D.; Nelyubina, Y.V.; Pavlov, A.A. Pathway bifurcations in the cage rearrangement of metallacarboranes: Experimental and computational evidence. Dalton Trans. 2021, 50, 287–293.
- Stogniy, M.Y.; Anufriev, S.A.; Bogdanova, E.V.; Sivaev, I.B.; Bregadze, V.I. Mercury(II) chloride in the synthesis of nido-carborane derivatives with B-N, B-O, and B-S bonds. Russ. Chem. Bull. 2022, 71, 91–101.
- Young, D.C.; Howe, D.V.; Hawthorne, M.F. Ligand derivatives of (3)-1,2-dicarbadodecahydroundecaborate(-1). J. Am. Chem. Soc. 1969, 91, 859–862.
- Meshcheryakov, V.I.; Kitaev, P.S.; Lyssenko, K.A.; Starikova, Z.A.; Petrovskii, P.V.; Janoušek, Z.; Corsini, M.; Laschi, F.; Zanello, P.; Kudinov, A.R. (Tetramethylcyclobutadiene)cobalt complexes with monoanionic carborane ligands − (L = SMe2, NMe3 and py). J. Organomet. Chem. 2005, 690, 4745–4754.
- Kang, H.C.; Lee, S.S.; Knobler, C.D.; Hawthorne, M.F. Syntheses of charge-compensated dicarbollide ligand precursors and their use in the preparation of novel metallacarboranes. Inorg. Chem. 1991, 30, 2024–2031.
- Zakharkin, L.I.; Kalinin, V.N.; Zhigareva, G.G. Oxidation of dicarbadodecahydro-nido-undecaborate anions by mercuric chloride in tetrahydrofuran and pyridine. Russ. Chem. Bull. 1979, 28, 2198–2199.
- Brattsev, V.A.; Danilova, G.N.; Stanko, V.I. Features of oxidative amination of o-dicarbaundecaborates. Zh. Obshch. Khim. 1972, 42, 1333–1339.
- Zakharkin, L.I.; Grandberg, N.V.; Antonovich, V.A. Synthesis of derivatives of (3)-1,2-dicarba-nido-undecaborate containing B-Si and B-P σ-bonds. Russ. Chem. Bull. 1976, 25, 1724–1727.
- Zakharkin, L.I.; Ol’shevskaya, V.A.; Zhigareva, G.G.; Antonovich, V.A.; Petrovskii, P.V.; Yanovskii, A.I.; Polyakov, A.V.; Struchkov, Y.T. The substitution reaction at boron in the pentagonal plane in 7,8-C2B9H12−K+ and 7,8-C2B9H112−2Na+ on treatment with chlorodiphenylphosphine in solution in tetrahydrofuran. Organomet. Chem. USSR 1989, 2, 671–676.
- Kim, K.-M.; Do, Y.-K.; Knobler, C.B.; Hawthorne, M.F. Synthesis and structural characterization of a zwitterionic triphenylphosphine derivative of the dicarbollide anion: . Bull. Korean Chem. Soc. 1989, 10, 321–322.
- Chen, M.; Zhao, D.; Xu, J.; Li, C.; Lu, C.; Yan, H. Electrooxidative B-H functionalization of nido-carboranes. Angew. Chem. Int. Ed. 2021, 60, 7838–7844.
- Yang, Z.; Sun, C.; Wei, X.; Lu, J.; Lu, J.-Y. Palladium-catalyzed cascade deboronation/regioselective B-P coupling of closo-carboranes. ChemCatChem 2022, 14, e202101571.
- Sivaev, I.B.; Prikaznov, A.V.; Anufriev, S.A. On relative electronic effects of polyhedral boron hydrides. J. Organomet. Chem. 2013, 747, 254–256.
- Stogniy, M.Y.U.; Sivaev, I.B. Synthesis and reactivity of cyclic oxonium derivatives of nido-carborane: A review. Reactions 2022, 3, 172–191.
- Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives as an efficient synthetic tool for the modification of polyhedral boron hydrides. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 623–637.
- Stogniy, M.Y.; Abramova, E.N.; Lobanova, I.A.; Sivaev, I.B.; Bragin, V.I.; Petrovskii, P.V.; Tsupreva, V.N.; Sorokina, O.V.; Bregadze, V.I. Synthesis of functional derivatives of 7,8-dicarba-nido-undecaborate anion by ring-opening of its cyclic oxonium derivatives. Collect. Czech. Chem. Commun. 2007, 72, 1676–1688.
- Frank, R.; Auer, H.; Hey-Hawkins, E. Functionalisation of the nido-dicarbaborate anion nido-7,8-C2B9H12− by hydride abstraction. J. Organomet. Chem. 2013, 747, 217–224.
- Plešek, J.; Jelínek, T.; Mareš, F.; Heřmánek, S. Unique dialkylsulfoniomethylation of the 7,8-C2B9H12− ion to the 9-R2S-CH2-7,8-C2B9H11 zwitterions by formaldehyde and dialkyl sulfides. General synthesis of the compounds 10-R2E-7,8-C2B9H11 (E. = O, S). Collect. Czech. Chem. Commun. 1993, 58, 1534–1547.
- Řezácová, P.; Pokorná, J.; Brynda, J.; Kožíšek, M.; Cígler, P.; Lepšík, M.; Fanfrlík, J.; Řezáč, J.; Šašková, K.G.; Sieglová, I.; et al. Design of HIV protease inhibitors based on inorganic polyhedral metallacarboranes. J. Med. Chem. 2009, 52, 7132–7141.
- Bakardjiev, M.; El Anwar, S.; Bavol, D.; Růžičková, Z.; Grüner, B. Focus on chemistry of the 10-dioxane-nido-7,8-dicarba-undecahydrido undecaborate zwitterion; exceptionally easy abstraction of hydrogen bridge and double-action pathways observed in ring cleavage reactions with OH− as nucleophile. Molecules 2020, 25, 814.
- Stogniy, M.Y.; Sivaev, I.B.; Malysheva, Y.B.; Bregadze, V.I. Synthesis of Tetrahydropyran Oxonium Derivative of 7,8-Dicarba-nido-undecaborane anion ; Vestnik N. I. Lobachevskiy Nizhegorod University: Nizhny Novgorod, Russia, 2013; Volume 4, pp. 115–117. Available online: http://www.unn.ru/pages/e-library/vestnik/99999999_West_2013_4(1)/19.pdf (accessed on 31 January 2023).
- Laskova, J.; Kosenko, I.; Serdyukov, A.; Sivaev, I.; Bregadze, V.I. Synthesis of naphthalimide derivatives of closo-dodecaborate and nido-carborane. J. Organomet. Chem. 2022, 959, 122186.
- Anufriev, S.A.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Laskova, J.N.; Godovikov, I.A.; de Biani, F.F.; Corsini, M.; Sivaev, I.B.; Bregadze, V.I. Synthesis and structure of bis(methylsulfanyl) derivatives of iron bis(dicarbollide). J. Organomet. Chem. 2018, 865, 239–256.
- Plešek, J.; Janoušek, Z.; Heřmánek, S. Four new (CH3)2S·C2B9H11 isomers. Collect. Czech. Chem. Commun. 1978, 43, 2862–2868.
- Yan, Y.-K.; Mingos, D.M.P.; Müller, T.E.; Williams, D.J.; Kurmoo, M. Synthesis and structure of a charge-compensated ferracarborane, commo-, and its charge-transfer salt with 2,3-dichloro-5,6-dicyano-p-benzoquinone. J. Chem. Soc. Dalton Trans. 1994, 1735–1741.
- Rosair, G.M.; Welch, A.J.; Weller, A.S.; Zahn, S.K. Sterically encumbered charge-compensated carbaboranes: Synthesis and reactivity. Molecular structures of 7-Ph-11-SMe2-7,8-nido-C2B9H10 and 1-Ph-3,3-(CO)2-7-SMe2-3,1,2-closo-RhC2B9H8. J. Organomet. Chem. 1997, 536–537, 299–308.
- Sivaev, I.B.; Prikaznov, A.V.; Naoufal, D. Fifty years of the closo-decaborate anion chemistry. Collect. Czech. Chem. Commun. 2010, 75, 1149–1199.
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. Chemistry of closo-dodecaborate anion 2−: A review. Collect. Czech. Chem. Commun. 2002, 67, 679–727.
- Vinogradov, M.M.; Nelyubina, Y.V.; Aliyeu, T.M. New aspects of acid-assisted nucleophilic substitution reactions of 11-vertex nido-carboranes. Polyhedron 2022, 214, 115654.
- Cowie, J.; Hamilton, E.J.M.; Laurie, J.C.V.; Welch, A.J. Structure of 10,11-μ-hydro-9-dimethylsulfido-7,8-dicarba-nido-undecaborane(11). Acta Cryst. 1988, C44, 1648–1650.
- Ellis, D.; Rosair, G.M.; Robertson, S.; Welch, A.J. 7,8-Di phenyl-9-di methyl sulfido-10,11-μ-hydro-7,8-dicarba-nido-undecaborane(9). Acta Cryst. 2000, C56, 1399–1400.
- Hendershot, S.L.; Jeffery, J.C.; Jelliss, P.A.; Mullica, D.F.; Sappenfield, E.L.; Stone, F.G.A. Reaction of nido-7,8-C2B9H13 with dicobalt octacarbonyl: Crystal structures of the complexes , , and . Inorg. Chem. 1996, 35, 6561–6570.
- Kuvshinova, S.S.; Nelyubina, Y.V.; Smol’yakov, A.F.; Kosenko, I.D.; Barakovskaya, I.G.; Loginov, D.A. Usage of (C5R5)Co(CO)I2 (R = H, Me) for the synthesis of 12-vertex closo-cobaltacarboranes. Unexpected formation of 10--7,8-Me2-7,8-nido-C2B9H9. J. Organomet. Chem. 2018, 865, 109–113.
More
