You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Vincenzo Ricci.
Lateral epicondylitis (LE) is a very common and painful condition seen in the daily practice of musculoskeletal physicians. Ultrasound-guided (USG) injections are commonly performed to manage the pain, promote the healing phase, and plan a tailored rehabilitation treatment. In this aspect, several techniques were described to target specific pain generators i the lateral elbow.
- elbow
- pain
- tendon
- ultrasonography
- intervention
1. Introduction
Lateral epicondylitis (LE) can be considered as an umbrella definition—commonly used to include several pathological conditions involving the extra-articular soft tissues of the lateral elbow compartment [1]. Indeed, not only the common extensor tendon (CET), but also the subcutaneous tissue, the forearm fascia, and the tendon-bone junction (i.e., enthesis of the lateral epicondyle) can contribute to this clinical scenario [1]. Moreover, despite the historical “itis” in its name, it is well known that a very poor presence of white blood cells can be demonstrated within the soft tissues of the lateral elbow in relevant patients [2]. Instead, an intratendinous invasion of immature fibroblasts and chaotic neurovascular tangles were histologically identified, i.e., the so-called angiofibroblastic hyperplasia [3,4,5][3][4][5]. Epidemiologically, prospective studies and meta-analyses seem to strongly support the association between wrist/elbow biomechanics and the incidence of LE [6]. This “lateral overload” theory includes strenuous manual tasks such as a combination of repetitive movements and prolonged postures. It should also be kept in mind that the biological imbalance due to multiple microtraumas might exceed the healing responses of the soft tissues.
Considering the multiple aforementioned (potential) pain generators and histopathological findings, several USG techniques were described/used to manage the lateral elbow pain as well as to plan for appropriate rehabilitation. Indeed, starting from the extremely variable sonographic findings encountered in patients with LE, a wide range of USG techniques can be used. Herewith, the pertinent literature also comprises incorrect and ‘historical’ terminology referring to those methods [7].
2. Peritendinous Injection
The dermo-epidermal complex, subcutaneous tissue, and forearm fascia wrap the lateral elbow compartment and, using a high-frequency linear probe, they can all easily be identified as superficial to the CET [8,9][8][9]. At this level, the recurrent radial artery, posterior branch of the radial collateral artery, and the interosseous recurrent artery anastomose—forming a superficial vascular plexus which covers the lateral epicondyle and the CET [10]. Interestingly, in patients with signs and symptoms of LE, a hypertrophic neurovascular network was histologically identified within the aforementioned superficial soft tissues which envelops and penetrates the superficial fibers of the CET [11]. This condition is known as superficial-to-deep vascular invasion of the tendon tissue (Figure 1)—with larger vascular elements being in the subcutaneous tissue (i.e., the donator vessels) and thinner vascular signals residing within the CET (i.e., the penetrating vessels) [1].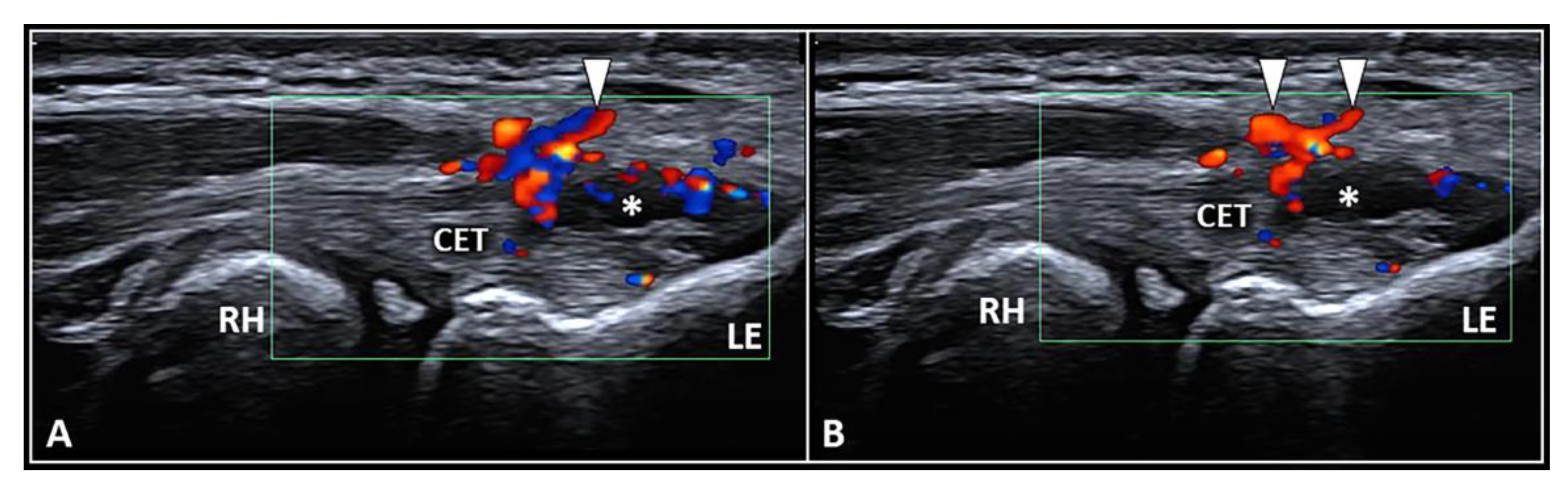
Figure 1. Ultrasound examination with color Doppler (A,B) clearly shows how the aberrant neovessels (white arrowheads) originating from the superficial vascular plexus of the subcutis pierce the forearm fascia invading the hypoechoic zone (white asterisk) of the CET related to insertional tendinosis. RH: radial head, LE: lateral epicondyle, CET: common extensor tendon.
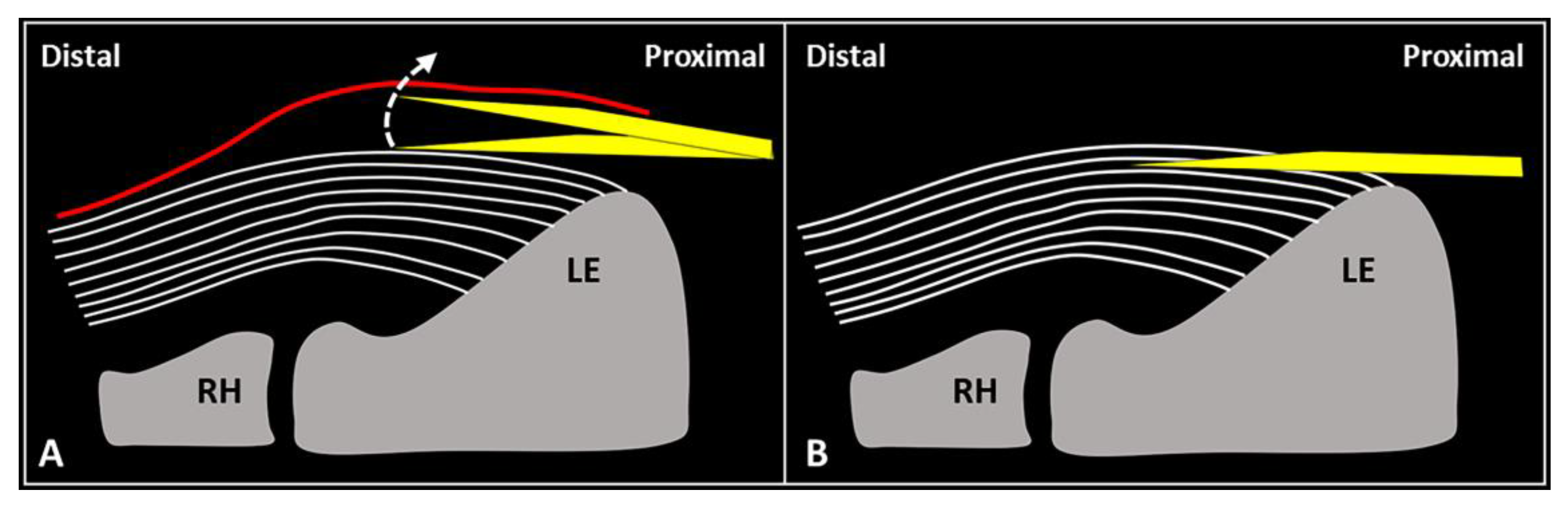
Figure 2. When the needle’s tip (yellow) is correctly positioned just over the superficial edge of the CET, by gently tilting (curved dotted arrow) the needle, it is possible to “separate” the forearm fascia (red) from the underlying tendon without hard resistance (A). Conversely, if the needle’s tip (yellow) is located inside the superficial portion of the CET, a “block” can be felt during this maneuver due to the intratendinous entrapment of the needle between the tendon fibers (white lines) (B). RH: radial head, LE: lateral epicondyle.
3. Intratendinous Injection
The CET is an anchor tendon, mainly composed of longitudinally oriented collagen fibers attaching to the lateral epicondyle of the humerus. Normally, it presents a hyperechoic fibrillar pattern quite similar to the underlying RCL [1]. The extensor carpi radialis brevis tendon represents its deepest layer whereas the extensor digitorum communis tendon constitutes the most superficial portion of the common tendinous mass [18][15]. Interestingly, extensor carpi radialis longus, extensor carpi ulnaris, and the CET are not fused with each other—presenting as separate anatomical structures [19][16]. The vascular network is not uniformly distributed within the CET and unlike the superficial layers perfused by a rich subcutaneous plexus, the deep portion is almost avascular [10]. Among several pathological conditions potentially involved in the clinical scenario of LE, focal tendinosis, partial tear, and intratendinous calcific deposition are the most commonly encountered in daily practice (Figure 3) [1].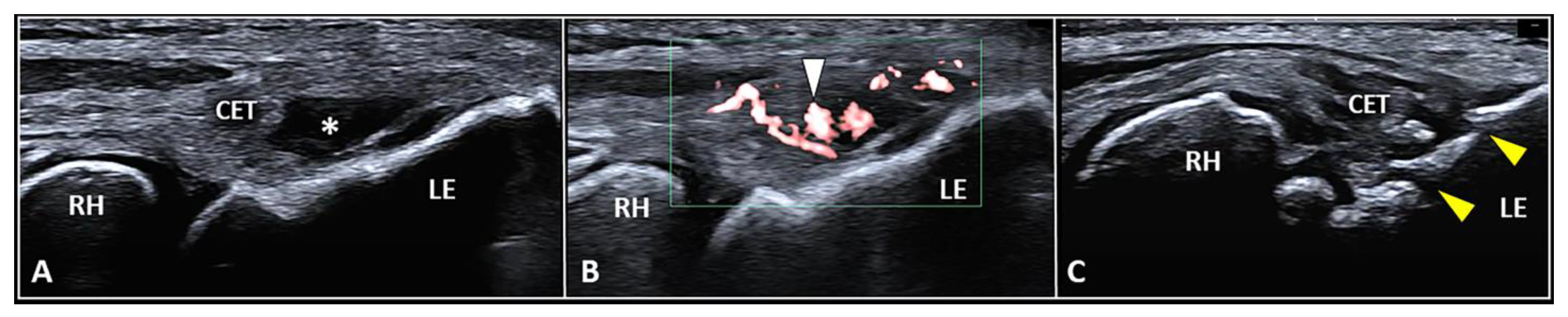
Figure 3. Partial tear (white asterisk) of the insertion zone of CET (A), focal tendinosis with inner hypervascularization (white arrowhead) (B), and enthesitis with cortical irregularities (yellow arrowhead) (C) of the lateral epicondyle (LE) should be considered among the most common sonographic findings related to the clinical picture of lateral epicondylitis. RH: radial head.
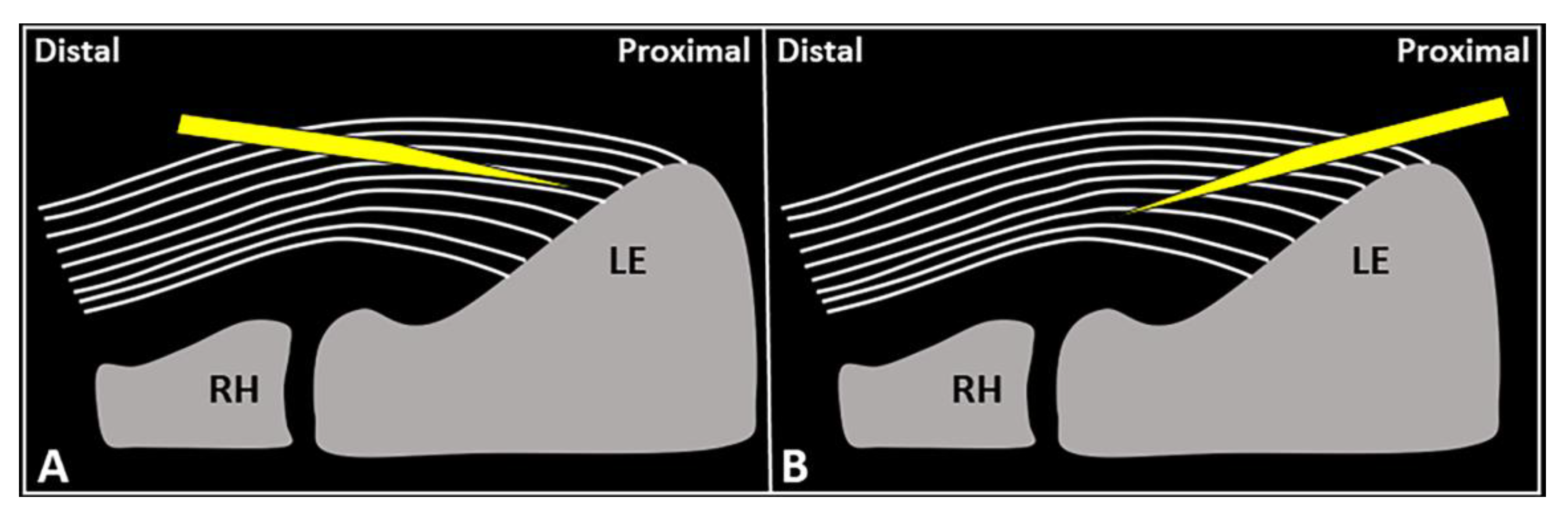
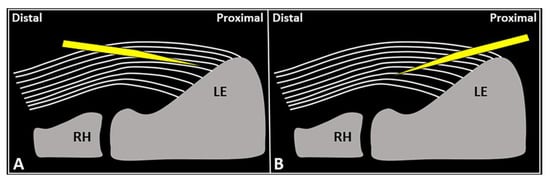
Figure 4.
Schematic drawing shows the distal-to-proximal (
A
) and proximal-to-distal (
B
) approaches for performing USG fenestration of the CET. While the needle tip (
yellow
) can slip in between the tendon fibers (
white lines
) in the former technique (
A
); crossing in an orthogonal fashion, it efficiently disrupts them in the latter (
B). RH: radial head, LE: lateral epicondyle.
). RH: radial head, LE: lateral epicondyle.
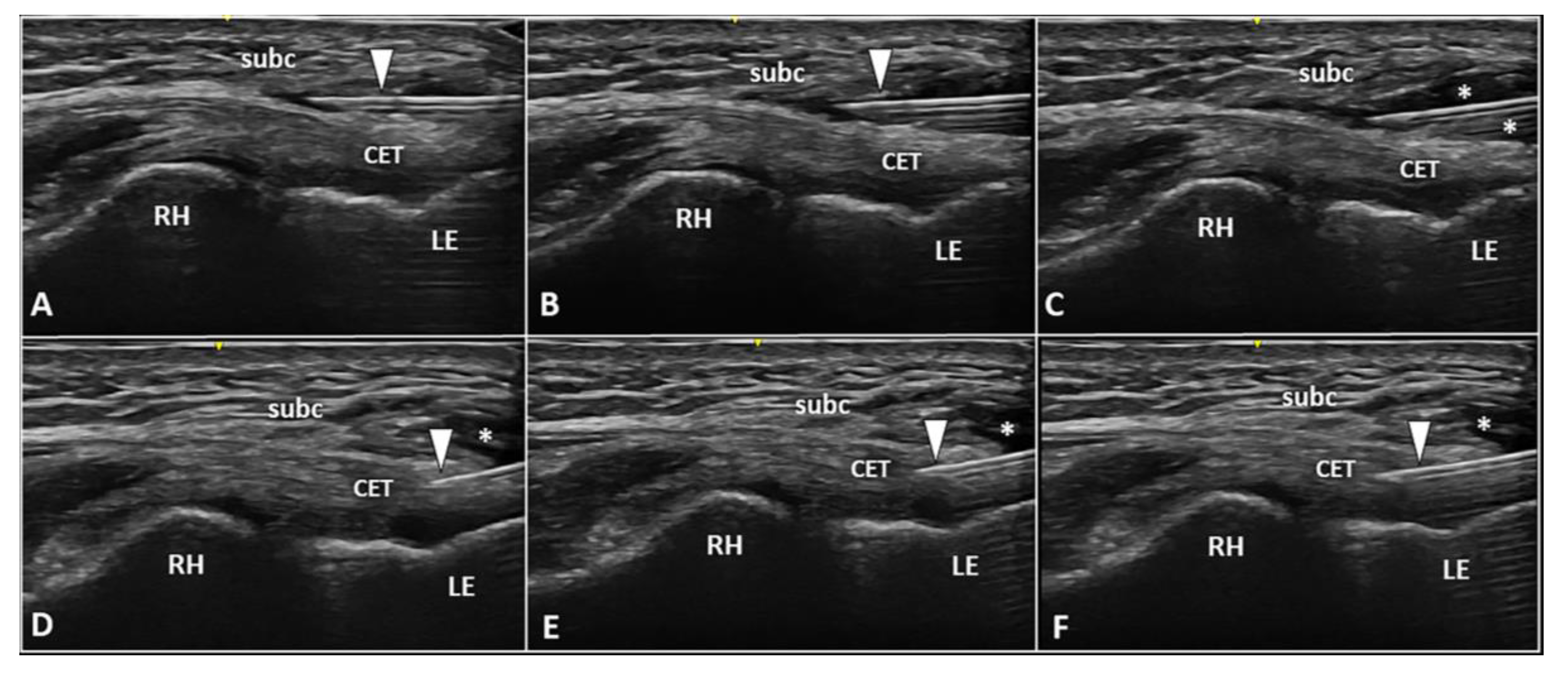
Figure 5. Two-step USG procedure of the lateral elbow using in-plane proximal-to-distal approach. The needle (white arrowhead) is advanced in between the subcutaneous tissue (subc) and the CET. High volume (white asterisks) is injected to efficiently “open” the pathological interface (A–C). The needle (white arrowhead) is redirected more deeply to perform the fenestration of the pre-insertional segment of CET (D–F). RH: radial head, LE: lateral epicondyle.
4. Enthesis Injection
The fibers of the CET anchor to the lateral epicondyle through a transitional plate known as enthesis. Histologically, it is a double-layer structure made of an uncalcified fibrocartilaginous stratum in continuum with the tendon fibers and a calcified fibrocartilage attached to the subchondral bone [36,37][31][32]. In physiological conditions, the sonographic appearance of lateral enthesis is similar to a smooth hypoechoic band located between the hyperechoic cortical surface of the lateral epicondyle and the regular fibrillar pattern of the CET. [1,38,39][1][33][34] Likewise, in patients with enthesopathies, focal interruptions of the cortical bone, pitting of the trabecular bone, lamellar calcifications of the fibrocartilaginous plate, and vascular signals at color/power Doppler are the most common sonographic findings (Figure 3) [1,38,39][1][33][34]. In the authors’ experience, the USG injection of the enthesis is less commonly performed when compared to peri/intratendinous injections in the daily management of patients with LE. Likewise, focal detachment (i.e., avulsion) of the CET fibers from the fibrocartilaginous plate of the lateral elbow can be considered a non-rare pathological condition requiring this intervention. Technically, using the in-plane distal-to-proximal approach, suggest a two-step procedure (Figure 6) with an intralesional injection of local anesthetic followed by abrasion of the fibrocartilage with rotations of the needle bevel causing a “drilling effect”.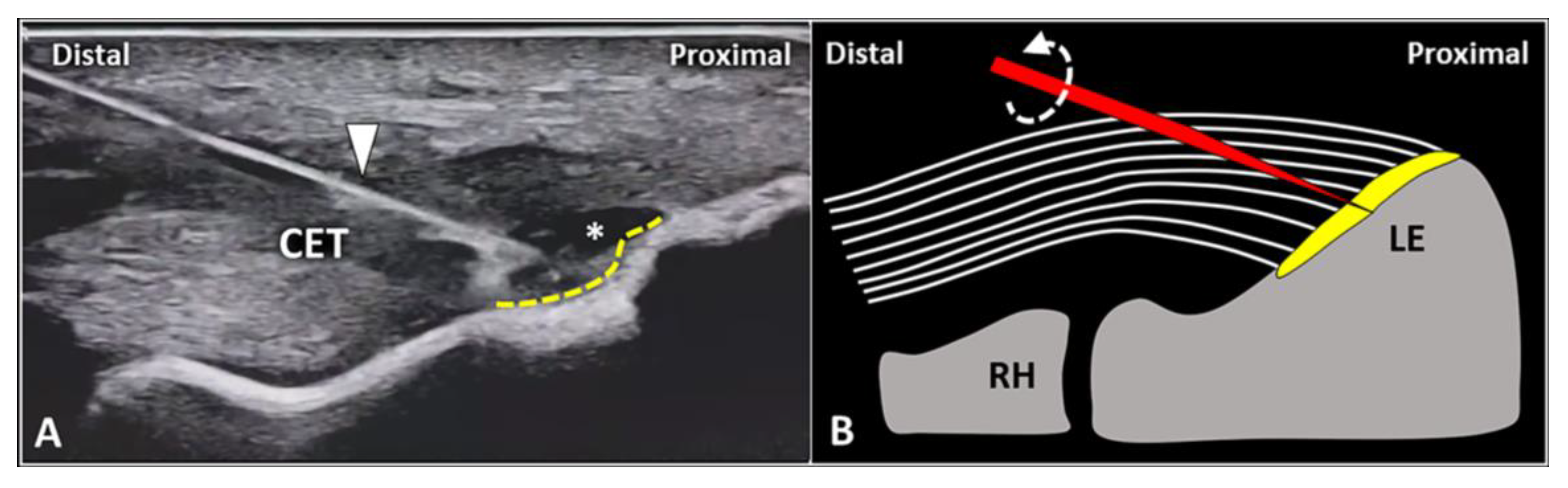
Figure 6. In a patient with focal avulsion of deep fibers of CET from the lateral epicondyle (LE), a two-step USG procedure can be performed. Using an in-plane distal-to-proximal approach, the needle (white arrowhead) is advanced within the injury (white asterisk) and local anesthetic is injected to “uncover” the underlying enthesis (yellow dotted line) (A). Schematic drawing shows multiple rotations (curved dotted arrow) of the needle’s tip (red) for mechanical drilling of the fibrocartilage (yellow) to induce a local micro-bleeding (B). RH: radial head.
References
- Ricci, V.; Cocco, G.; Mezian, K.; Chang, K.V.; Naňka, O.; Tamborrini, G.; Kara, M.; Özçakar, L. Anatomy and sonographic examination for lateral epicondylitis: EURO-MUSCULUS/USPRM* approach. Am. J. Phys. Med. Rehabil. 2022.
- Binder, A.I.; Hazleman, B.L. Lateral humeral epicondylitis–a study of natural history and the effect of conservative therapy. Br. J. Rheumatol. 1983, 22, 73–76.
- Nirschl, R.P.; Ashman, E.S. Elbow tendinopathy: Tennis elbow. Clin. Sports Med. 2003, 22, 813–836.
- Zeisig, E.; Ohberg, L.; Alfredson, H. Extensor origin vascularity related to pain in patients with Tennis elbow. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 659–663.
- Potter, H.G.; Hannafin, J.A.; Morwessel, R.M.; DiCarlo, E.F.; O’Brien, S.J.; Altchek, D.W. Lateral epicondylitis: Correlation of MR imaging, surgical, and histopathologic findings. Radiology 1995, 196, 43–46.
- Descatha, A.; Albo, F.; Leclerc, A.; Carton, M.; Godeau, D.; Roquelaure, Y.; Petit, A.; Aublet-Cuvelier, A. Lateral epicondylitis and physical exposure at work? A review of prospective studies and meta-analysis. Arthritis Care Res. 2016, 68, 1681–1687.
- Hall, M.M.; Allen, G.M.; Allison, S.; Craig, J.; DeAngelis, J.P.; Delzell, P.B.; Finnoff, J.T.; Frank, R.M.; Gupta, A.; Hoffman, D.; et al. Recommended musculoskeletal and sports ultrasound terminology: A delphi-based consensus statement. Br. J. Sports Med. 2022, 56, 310–319.
- Ricci, V.; Ricci, C.; Cocco, G.; Donati, D.; Farì, G.; Mezian, K.; Naňka, O.; Özçakar, L. From histology to sonography in skin and superficial tissue disorders: EURO-MUSCULUS/USPRM* approach. Pathol. Res. Pract. 2022, 237, 154003.
- Ricci, V.; Ricci, C.; Gervasoni, F.; Andreoli, A.; Özçakar, L. From histo-anatomy to sonography in lymphedema: EURO-MUSCULUS/USPRM approach. Eur. J. Phys. Rehabil. Med. 2022, 58, 108–117.
- Schneeberger, A.G.; Masquelet, A.C. Arterial vascularization of the proximal extensor carpi radialis brevis tendon. Clin. Orthop. Relat. Res. 2002, 398, 239–244.
- Spang, C.; Alfredson, H. Richly innervated soft tissues covering the superficial aspect of the extensor origin in patients with chronic painful tennis elbow—Implication for treatment? J. Musculoskelet. Neuronal Interact. 2017, 17, 97–103.
- Ricci, V.; Ricci, C.; Tamborrini, G.; Chang, K.V.; Mezian, K.; Zunica, F.; Naňka, O.; Kara, M.; Özçakar, L. From histology to sonography in synovitis: EURO-MUSCULUS/USPRM approach. Pathol. Res. Pract. 2023, 241, 154273.
- Ricci, V.; Abdulsalam, A.J.; Özçakar, L. Ultrasound Imaging for Dummies: Getting oriented among the planes. J. Rehabil. Med. 2019, 51, 624–625.
- Ricci, V.; Özçakar, L. From “ultrasound imaging” to “ultrasound examination”: A needful upgrade in musculoskeletal medicine. Pain Med. 2020, 21, 1304–1306.
- Clarke, A.W.; Ahmad, M.; Curtis, M.; Connell, D.A. Lateral elbow tendinopathy: Correlation of ultrasound findings with pain and functional disability. Am. J. Sports Med. 2010, 38, 1209–1214.
- Greenbaum, B.; Itamura, J.; Vangsness, C.T.; Tibone, J.; Atkinson, R. Extensor carpi radialis brevis. An anatomical analysis of its origin. J. Bone Jt. Surg. Br. 1999, 81, 926–929.
- Zeisig, E.; Ohberg, L.; Alfredson, H. Sclerosing polidocanol injections in chronic painful tennis elbow-promising results in a pilot study. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 1218–1224.
- Ljung, B.O.; Forsgren, S.; Fridén, J. Substance P and calcitonin gene-related peptide expression at the extensor carpi radialis brevis muscle origin: Implications for the etiology of tennis elbow. J. Orthop. Res. 1999, 17, 554–559.
- Krogh, T.P.; Fredberg, U.; Ammitzbøll, C.; Ellingsen, T. Clinical value of ultrasonographic assessment in lateral epicondylitis versus asymptomatic healthy controls. Am. J. Sports Med. 2020, 48, 1873–1883.
- du Toit, C.; Stieler, M.; Saunders, R.; Bisset, L.; Vicenzino, B. Diagnostic accuracy of power doppler ultrasound in patients with chronic tennis elbow. Br. J. Sports Med. 2008, 42, 872–876.
- Ricci, V.; Güvener, O.; Chang, K.V.; Wu, W.T.; Mezian, K.; Kara, M.; Leblebicioğlu, G.; Pirri, C.; Ata, A.M.; Dughbaj, M.; et al. EURO-MUSCULUS/USPRM dynamic ultrasound protocols for elbow. Am. J. Phys. Med. Rehabil. 2022, 101, e83–e92.
- Sussman, W.I.; Williams, C.J.; Mautner, K. Ultrasound-guided elbow procedures. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 573–587.
- Ricci, V.; Schroeder, A.; Özçakar, L. Ultrasound imaging for lateral elbow pain: Pinpointing the epicondylosis. Am. J. Phys. Med. Rehabil. 2020, 99, 560–561.
- De Maeseneer, M.; Brigido, M.K.; Antic, M.; Lenchik, L.; Milants, A.; Vereecke, E.; Jager, T.; Shahabpour, M. Ultrasound of the elbow with emphasis on detailed assessment of ligaments, tendons, and nerves. Eur. J. Radiol. 2015, 84, 671–681.
- Park, G.Y.; Kwon, D.R.; Cho, H.K.; Park, J.; Park, J.H. Distribution of platelet-rich plasma after ultrasound-guided injection for chronic elbow tendinopathies. J. Sports Sci. Med. 2017, 16, 1–5.
- Hammerman, M.; Aspenberg, P.; Eliasson, P. Microtrauma stimulates rat achilles tendon healing via an early gene expression pattern similar to mechanical loading. J. Appl. Physiol. (1985) 2014, 116, 54–60.
- Darrieutort-Laffite, C.; Soslowsky, L.J.; Le Goff, B. Molecular and structural effects of percutaneous interventions in chronic achilles tendinopathy. Int. J. Mol. Sci. 2020, 21, 7000.
- Calderón-Díez, L.; Sánchez-Sánchez, J.L.; Herrero-Turrión, J.; Cleland, J.; Arias-Buría, J.L.; Fernández-de-Las-Peñas, C. Dry needling of a healthy rat achilles tendon increases its gene expressions: A pilot study. Pain Med. 2021, 22, 112–117.
- Stoychev, V.; Finestone, A.S.; Kalichman, L. Dry needling as a treatment modality for tendinopathy: A narrative review. Curr. Rev. Musculoskelet. Med. 2020, 13, 133–140.
- Pringels, L.; Cook, J.L.; Witvrouw, E.; Burssens, A.; Vanden Bossche, L.; Wezenbeek, E. Exploring the role of intratendinous pressure in the pathogenesis of tendon pathology: A narrative review and conceptual framework. Br. J. Sports Med. 2022.
- Milz, S.; Tischer, T.; Buettner, A.; Schieker, M.; Maier, M.; Redman, S.; Emery, P.; McGonagle, D.; Benjamin, M. Molecular composition and pathology of entheses on the medial and lateral epicondyles of the humerus: A structural basis for epicondylitis. Ann. Rheum. Dis. 2004, 63, 1015–1021.
- Kehl, A.S.; Corr, M.; Weisman, M.H. Review: Enthesitis: New insights into pathogenesis, diagnostic modalities, and treatment. Arthritis Rheumatol. 2016, 68, 312–322.
- Tamborrini, G.; Bruyn, G.A. CME-Sonografie 93: Ultraschall der Enthese—Nicht jede «Enthesitis» bedeutet eine Spondyloarthritis . Praxis 2020, 109, 888–896.
- Gandjbakhch, F.; Terslev, L.; Joshua, F.; Wakefield, R.J.; Naredo, E.; D’Agostino, M.A.; OMERACT Ultrasound Task Force. Ultrasound in the evaluation of enthesitis: Status and perspectives. Arthritis Res. Ther. 2011, 13, R188.
- Ricci, V.; Becciolini, M.; Özçakar, L. Ultrasound imaging for recalcitrant lateral elbow pain: Radio-humeral synovial plica is also at play. Pain Med. 2020, 21, 875–878.
- Ricci, V.; Özçakar, L. Ultrasound-guided intra-articular injection of the elbow: Targeting deep to anconeus muscle. Pain Med. 2020, 21, 3242–3243.
- Tortora, S.; Messina, C.; Albano, D.; Serpi, F.; Corazza, A.; Carrafiello, G.; Sconfienza, L.M.; Gitto, S. Ultrasound-guided musculoskeletal interventional procedures around the elbow, hand and wrist excluding carpal tunnel procedures. J. Ultrason. 2021, 21, e169–e176.
- Chen, J.L.; Cheng, W.J.; Chen, K.Y.; Chen, C.P.C. Ultrasound-guided injection approach of treating tennis elbow and elbow joint synovitis simultaneously under one needle insertion point. Am. J. Phys. Med. Rehabil. 2022, 101, e115–e116.
- Kalichman, L.; Bannuru, R.R.; Severin, M.; Harvey, W. Injection of botulinum toxin for treatment of chronic lateral epicondylitis: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2011, 40, 532–538.
- Galván Ruiz, A.; Vergara Díaz, G.; Rendón Fernández, B.; Echevarría Ruiz De Vargas, C. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxin A) in patients with lateral epicondylitis. Toxins 2019, 11, 46.
More
