The cell-free DNA (cfDNA) levels are known to increase in biological fluids in various pathological conditions. However, the data on circulating cfDNA in severe psychiatric disorders, including schizophrenia, bipolar disorder (BD), and depressive disorders (DDs), is contradictory. The meta-analysis showed that the levels of total cfDNA and genomic cfDNA in patients with schizophrenia are significantly higher than in healthy donors (SMD values of 0.61 and 0.6, respectively; p < 0.00001). Data on mitochondrial cfDNA in schizophrenia were scarce. Meta-analysis in BD and DDs found no significant differences in the level of mitochondrial cfDNA. However, further research on mitochondrial and genomic cfDNA levels in psychiatric disorders is needed due to the data heterogeneity.
- cfDNA
- cf-mtDNA
- DAMPs
- schizophrenia
- bipolar disorder
- major depressive disorder
1. Introduction
2. Reports Characteristics
The list of reports included in the meta-analysis and their characteristics are summarized in Table 1. Various analytical methods allow the detection of different types of cfDNA (cf-mtDNA and cf-gDNA) or total cfDNA. Therefore, researchers divided all reports depending on the type of analyzed cfDNA. As a result, the total cfDNA was investigated in 6 studies [37,38,39,40,41,42][20][21][22][23][24][25] and cf-gDNA in three reports in the case of schizophrenia [24,36,42][25][26][27]. There were insufficient data for a meta-analysis of cf-mtDNA in schizophrenia (only two studies) [34,35][28][29]. Cf-mtDNA was analyzed in BD and DDs. The data for other types of cfDNA were insufficient for a meta-analysis in BD and DDs. In particular, only one study on cf-gDNA was found for DDs [36][27]. Accordingly, this meta-analysis analyzed the total cfDNA and cf-gDNA in schizophrenia, and cf-mtDNA was investigated in BD and DDs.| Study | Year | DNA Type | Sample | Extraction Method | Detection Method | Population | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia | |||||||||||
| Ershova et al. [39] | Ershova et al. [22] | 2017 | Total CfDNA | Plasma | Solvent extraction method | FL, PicoGreen dye | SZ 58/HC 30 | ||||
| Jiang et al. [38] | Jiang et al. [21] | 2018 | Total CfDNA | Plasma | TIANamp Micro DNA Kit (spin-column) | FCS | SZ 65/HC 62 | ||||
| Ershova et al. [37] | Ershova et al. [20] | 2019 | Total CfDNA | Plasma | Solvent extraction method | FL, PicoGreen dye | SZ 100/HC 96 | ||||
| Jestkova et al. [40] | Jestkova et al. [23] | 2021 | Total CfDNA | Plasma | Solvent extraction method | FL, PicoGreen dye | SZ 334/HC 95 | ||||
| Ershova et al. [41] | Ershova et al. [24] | 2022 | Total CfDNA | Plasma | Solvent extraction method | FL, PicoGreen dye | SZ 100/HC 60 | ||||
| Lubotzky et al. [42] | Lubotzky et al. [25] | 2022 | Total CfDNA and Cf-gDNA | Plasma | QIAsymphony DSP Circulating DNA Kit (magnetic particles) | FL, bisulfite DNA treatment, PCR amplification followed by NGS | FEP 29/HC 31 | ||||
| Chen et al. [24] | Chen et al. [26] | 2021 | Cf-gDNA | Serum | TianLong DNA Kit (spin-column) | qPCR, target: Alu repeats | SZ 174/HC 100 | ||||
| Qi et al. [36] | Qi et al. [27] | 2020 | Cf-gDNA | Serum | TianLong DNA Kit (spin-column) | qPCR, target: Alu repeats | SZ 164/HC 100 | ||||
| Bipolar Disorder | |||||||||||
| Stertz et al. [25] | Stertz et al. [30] | 2015 | Cf-mtDNA | Serum | QIAmp DNA Mini Kit (spin-column) | qPCR, target: | MT-ATP8 | gene | BD 20/HC 20 | ||
| Kageyama et al. [35] | Kageyama et al. [29] | 2018 | Cf-mtDNA | Plasma | QIAamp DNA Blood Mini Kit (spin-column) | qPCR, target: | MT-ND1 | and | MT-ND4 | genes | BD 28/HC 29 |
| Jeong et al. [43] | Jeong et al. [31] | 2020 | Cf-mtDNA | Serum | QIAmp DNA Mini Kit (spin-column) | qPCR, target: | MT-ND1 | gene | BD 64/HC 41 | ||
| Kageyama et al. [44] | Kageyama et al. [32] | 2022 | Cf-mtDNA | Plasma | QIAamp DNA Blood Mini Kit (spin-column) | qPCR, target: | MT-ND1 | and | MT-ND4 | genes | BD 10/HC 10 |
| Depressive disorders | |||||||||||
| Lindqvist et al. [45] | Lindqvist et al. [33] | 2016 | Cf-mtDNA | Plasma | QIAmp 96 DNA Blood Kit (spin-column) | qPCR, target: | MT-ND2 | gene | Suicide attempters 37/HC 37 | ||
| Kageyama et al. [35] | Kageyama et al. [29] | 2018 | Cf-mtDNA | Plasma | QIAamp DNA Blood Mini Kit (spin-column) | qPCR, target: | MT-ND1 | and | MT-ND4 | genes | MDD 109/HC 29 |
| Lindqvist et al. [47] | Lindqvist et al. [34] | 2018 | Cf-mtDNA | Plasma | QIAmp 96 DNA Blood Kit (spin-column) | qPCR, target: | MT-ND1 | and | MT-ND4 | genes | MDD 50/HC 55 |
| Fernström et al. [46] | Fernström et al. [35] | 2021 | Cf-mtDNA | Plasma | QIAmp DNA Blood Mini Kit (spin-column) | qPCR, target: | MT-ND2 | gene | Current depression 236/HC 49 | ||
| Behnke et al. [48] | Behnke et al. [36] | 2022 | Cf-mtDNA | Serum | QIAamp DNA Micro Kit (spin-column) | qPCR with multiple target | MDD 24/HC 20 | ||||
3. CfDNA Level in Schizophrenia
As stated above, the total cfDNA and cf-gDNA were analyzed in schizophrenia due to insufficient data existing for another type of cfDNA (cf-mtDNA). A meta-analysis of the circulating total cfDNA in schizophrenia pooled data from six studies with a total of 686 patients and 375 healthy controls. It has been shown that the circulating total cfDNA concentration in schizophrenia is significantly higher than in healthy donors (Figure 1a). The SMD for the overall effect was 0.61 (95% CI = [0.40 to 0.82]), with moderate heterogeneity (Chi2 = 11.6, df = 5, p < 0.04; I2 = 57%). The test for the overall effect also confirmed the significance of the differences (Z = 5.6, p < 0.00001). No evidence of publication bias was observed using Egger’s test (p = 0.773) and Begg’s test (p = 0.851). The funnel plot analysis showed signs of asymmetry. The three reports on the left side of the graph were from the same research group using a similar method [37,39,41][20][22][24]. Therefore, the observed asymmetry may indicate a publication bias. One report falling outside the confidence interval was probably related to the measurement methodology (fluorescence correlation spectroscopy) [38][21]. Additionally, there was sample heterogeneity (one of the six reports analyzed FEP patients) [42][25].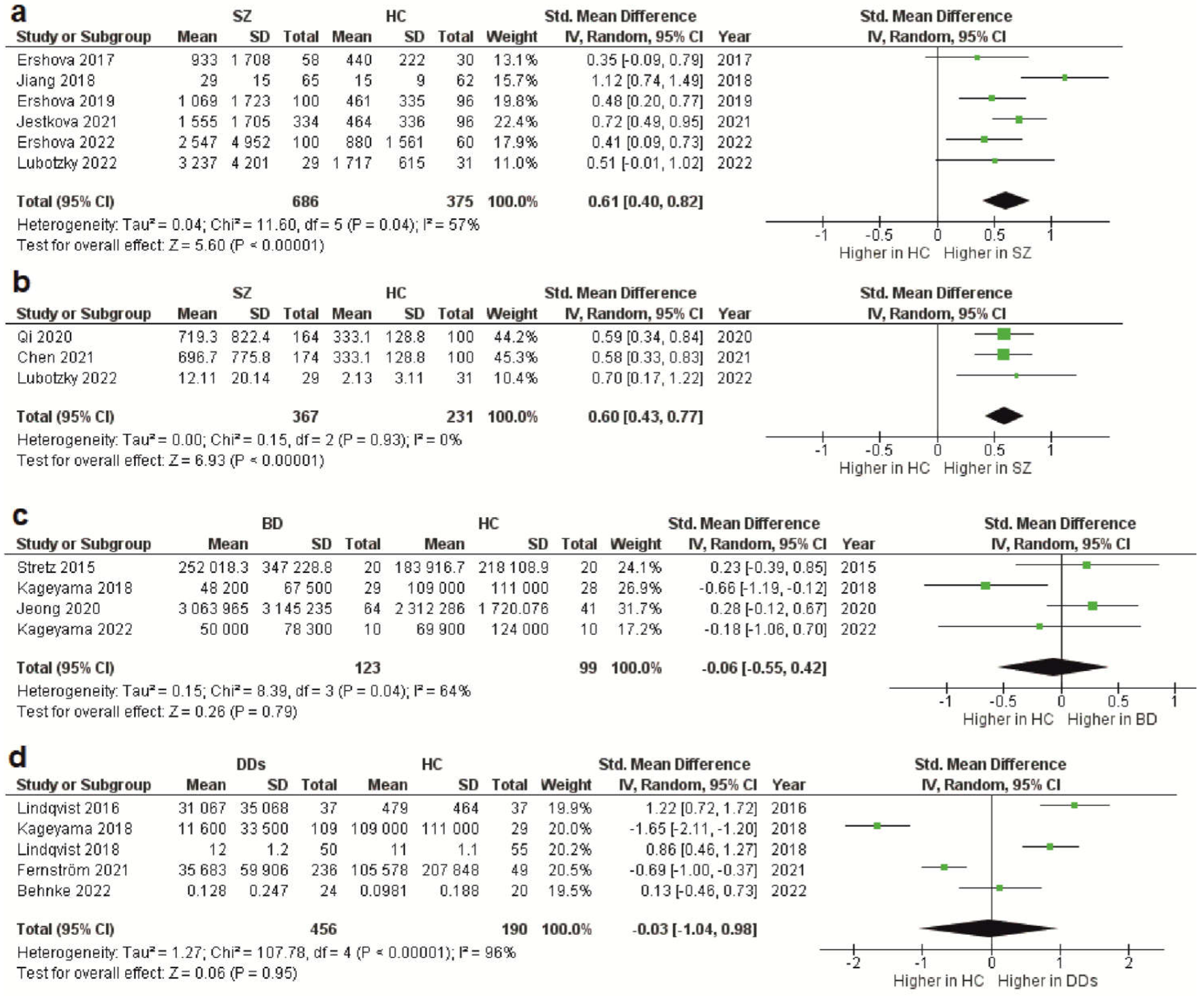 Figure 1. Forest plot showing plasma and serum levels of total cfDNA in patients with schizophrenia (a), cf-gDNA in schizophrenia (b), cf-mtDNA in BD (c), and cf-mtDNA in DDs (d) compared with healthy controls. BD—bipolar disorder; CI—confidence interval; DDs—depressive disorders; SD—standard deviation; SZ—schizophrenia; HC—healthy controls.
The meta-analysis of circulating cf-gDNA in schizophrenia included three studies with a total of 367 patients and 231 healthy individuals. A meta-analysis showed that the cf-gDNA concentration in schizophrenia was significantly higher than in the controls (Figure 1b). The SMD for the overall effect was 0.6 (95% CI = [0.43 to 0.77]). The test for the overall effect also confirmed the significance of the differences (Z = 6.93, p < 0.00001). Interestingly, there was practically no heterogeneity in the report results (Chi2 = 0.15, df = 2, p < 0.93; I2 = 0%). However, while Egger’s test (p = 0.058) and Begg’s test (p = 0.117) showed no evidence of bias, there were some indications of publication bias. In particular, the two reports had the same mean and standard deviation for the cf-gDNA for the group of healthy donors [24,36][26][27]. The funnel plot also confirmed this observation. Therefore, these results must be interpreted with caution. Nevertheless, after removing one of the studies with the same group of healthy donors [24][26], the meta-analysis results remained significant (SMD = 0.61, 95% CI = [0.38 to 0.84] with no heterogeneity (Chi2 = 0.13, df = 1, p < 0.72; I2 = 0%); test for overall effect: Z = 5.23, p < 0.00001). Therefore, further studies are needed to confirm the increased circulating cf-gDNA concentrations in schizophrenia.
Figure 1. Forest plot showing plasma and serum levels of total cfDNA in patients with schizophrenia (a), cf-gDNA in schizophrenia (b), cf-mtDNA in BD (c), and cf-mtDNA in DDs (d) compared with healthy controls. BD—bipolar disorder; CI—confidence interval; DDs—depressive disorders; SD—standard deviation; SZ—schizophrenia; HC—healthy controls.
The meta-analysis of circulating cf-gDNA in schizophrenia included three studies with a total of 367 patients and 231 healthy individuals. A meta-analysis showed that the cf-gDNA concentration in schizophrenia was significantly higher than in the controls (Figure 1b). The SMD for the overall effect was 0.6 (95% CI = [0.43 to 0.77]). The test for the overall effect also confirmed the significance of the differences (Z = 6.93, p < 0.00001). Interestingly, there was practically no heterogeneity in the report results (Chi2 = 0.15, df = 2, p < 0.93; I2 = 0%). However, while Egger’s test (p = 0.058) and Begg’s test (p = 0.117) showed no evidence of bias, there were some indications of publication bias. In particular, the two reports had the same mean and standard deviation for the cf-gDNA for the group of healthy donors [24,36][26][27]. The funnel plot also confirmed this observation. Therefore, these results must be interpreted with caution. Nevertheless, after removing one of the studies with the same group of healthy donors [24][26], the meta-analysis results remained significant (SMD = 0.61, 95% CI = [0.38 to 0.84] with no heterogeneity (Chi2 = 0.13, df = 1, p < 0.72; I2 = 0%); test for overall effect: Z = 5.23, p < 0.00001). Therefore, further studies are needed to confirm the increased circulating cf-gDNA concentrations in schizophrenia.
4. CfDNA Level in Bipolar Disorder
Less reliable data were obtained for BD because only four studies were included in the meta-analysis, with a total of 123 patients and 99 healthy controls. Only the cf-mtDNA concentration was analyzed in a meta-analysis. The results indicated that the circulating cf-mtDNA concentration in BD is not statistically different from in healthy donors (Figure 1c). The SMD for the overall effect was −0.06 (95% CI = [−0.55 to 0.42]), with moderate heterogeneity (Chi2 = 8.39, df = 3, p < 0.04; I2 = 64%). The test for the overall effect also showed no significant differences (Z = 0.06, p = 0.95). No evidence of publication bias was observed using Egger’s test (p = 0.646) and Begg’s test (p = 0.467). However, one report in the funnel plot was outside the confidence interval [35][29], which may indicate a publication bias. Thus, more research is needed on cf-mtDNA and other types of cfDNA in BD.5. CfDNA Level in Depressive Disorders
The meta-analysis in DDs included only data for the circulating cf-mtDNA. A meta-analysis pooled data from five studies with a total of 456 patients and 190 healthy controls. It has been shown that the cf-mtDNA concentrations in DDs are not significantly different from those in healthy donors (Figure 1d). The SMD for the overall effect was −0.03 (95% CI = [−1.04; 0.98]). It is important to note that substantial heterogeneity was observed (Chi2 = 107.78, df = 4, p < 0.00001; I2 = 96%). Egger’s test (p = 0.622) and Begg’s test (p = 0.2) showed no signs of bias. However, the funnel plot showed signs of publication bias, as many of the studies were outside the confidence intervals.6. Conclusion
In summary, this meta-analysis provided evidence for high levels of total cfDNA and cf-gDNA in the plasma and serum of patients with schizophrenia compared with healthy individuals. In contrast, evidence for altered cf-mtDNA levels in BD and DDs was not found in this meta-analysis. However, the lack of significant differences may be partly caused by the small number of studies in BD and the hypothetical publication bias in DDs. Data on other types of cfDNA or total cfDNA in these psychiatric disorders are scarce. In particular, there are few studies on cf-mtDNA in schizophrenia or total cfDNA and cf-gDNA in BD and DDs. Therefore, further research should fill this knowledge gap. High levels of cfDNA in schizophrenia may be associated with chronic systemic inflammation in schizophrenia, as cfDNA has been found to trigger inflammatory responses.References
- Aucamp, J.; Abel, J.B.; Christoffel, P.S.B. The diverse origins of circulating cell-free DNA in the human body: A critical re-evaluation of the literature. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1649–1683.
- Labbe, M.; Petresco, M.; Fabrykant, M.L. Taux du phosphore sanguin et de ses differentes formes dans les leucemies et les anemies. J. Soc. Biol. 1931, 107, T2.
- Mandel, P.; Metais, P. Nuclear acids in human blood plasma. Comptes Rendus Seances Soc. Biol. Fil. 1948, 142, 241–243.
- Bronkhorst, A.J.; Ungerer, V.; Diehl, F.; Anker, P.; Dor, Y.; Fleischhacker, M.; Gahan, P.B.; Hui, L.; Holdenrieder, S.; Thierry, A.R. Towards systematic nomenclature for cell-free DNA. Hum. Genet. 2021, 140, 565–578.
- Pös, O.; Biró, O.; Szemes, T.; Nagy, B. Circulating cell-free nucleic acids: Characteristics and applications. Eur. J. Hum. Genet. 2018, 26, 937–945.
- Grabuschnig, S.; Bronkhorst, A.J.; Holdenrieder, S.; Rosales, R.I.; Schliep, K.P.; Schwendenwein, D.; Ungerer, V.; Sensen, C.W. Putative origins of cell-free DNA in humans: A review of active and passive nucleic acid release mechanisms. Int. J. Mol. Sci. 2020, 21, 8062.
- Fernando, M.R.; Jiang, C.; Krzyzanowski, G.D.; Ryan, W.L. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS ONE 2017, 12, e0183915.
- Heitzer, E.; Auinger, L.; Speicher, M.R. Cell-free DNA and apoptosis: How dead cells inform about the living. Trends Mol. Med. 2020, 26, 519–528.
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794.
- Moss, J.; Magenheim, J.; Neiman, D.; Zemmour, H.; Loyfer, N.; Korach, A.; Samet, Y.; Maoz, M.; Druid, H.; Arner, P.; et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat. Commun. 2018, 9, 5068.
- Lo, Y.M.D.; Han, D.S.C.; Jiang, P.; Chiu, R.W.K. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 2021, 372, eaaw3616.
- Van der Meij, K.R.M.; Sistermans, E.A.; Macville, M.V.E.; Stevens, S.J.C.; Bax, C.J.; Bekker, M.N.; Bilardo, C.M.; Boon, E.M.J.; Boter, M.; Diderich, K.E.M.; et al. TRIDENT-2: National implementation of genome-wide non-invasive prenatal testing as a first-tier screening test in the Netherlands. Am. J. Hum. Genet. 2019, 105, 1091–1101.
- Fettke, H.; Kwan, E.M.; Azad, A.A. Cell-free DNA in cancer: Current insights. Cell. Oncol. (Dordr.) 2019, 42, 13–28.
- De Vlaminck, I.; Valantine, H.A.; Snyder, T.M.; Strehl, C.; Cohen, G.; Luikart, H.; Neff, N.F.; Okamoto, J.; Bernstein, D.; Weisshaar, D.; et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci. Transl. Med. 2014, 6, 241ra77.
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. 2019, 15, 493–518.
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112.
- Ermakov, E.A.; Melamud, M.M.; Buneva, V.N.; Ivanova, S.A. Immune System Abnormalities in Schizophrenia: An Integrative View and Translational Perspectives. Front. Psychiatry 2022, 13, 880568.
- Pereira, A.C.; Oliveira, J.; Silva, S.; Madeira, N.; Pereira, C.M.F.; Cruz, M.T. Inflammation in Bipolar Disorder (BD): Identification of new therapeutic targets. Pharmacol. Res. 2021, 163, 105325.
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34.
- Ershova, E.S.; Jestkova, E.M.; Martynov, A.V.; Shmarina, G.V.; Umriukhin, P.E.; Bravve, L.V.; Zakharova, N.V.; Kostyuk, G.P.; Saveliev, D.V.; Orlova, M.D.; et al. Accumulation of circulating cell-free CpG-enriched ribosomal DNA fragments on the background of high endonuclease activity of blood plasma in schizophrenic patients. Int. J. Genom. 2019, 2019, 8390585.
- Jiang, J.; Chen, X.; Sun, L.; Qing, Y.; Yang, X.; Hu, X.; Yang, C.; Xu, T.; Wang, J.; Wang, P.; et al. Analysis of the concentrations and size distributions of cell-free DNA in schizophrenia using fluorescence correlation spectroscopy. Transl. Psychiatry 2018, 8, 104.
- Ershova, E.S.; Jestkova, E.M.; Chestkov, I.V.; Porokhovnik, L.N.; Izevskaya, V.L.; Kutsev, S.I.; Veiko, N.N.; Shmarina, G.; Dolgikh, O.; Kostyuk, S.V. Quantification of cell-free DNA in blood plasma and DNA damage degree in lymphocytes to evaluate dysregulation of apoptosis in schizophrenia patients. J. Psychiatr. Res. 2017, 87, 15–22.
- Jestkova, E.M.; Ershova, E.S.; Martynov, A.V.; Zakharova, N.V.; Kostyuk, G.P.; Veiko, N.N.; Kostyuk, S.V. Concentration of Circulating Cell-Free DNA in the Peripheral Blood Plasma of Patients with Acute Endogenous and Exogenous Etiology Psychoses. Psikhiatria 2021, 19, 6–14.
- Ershova, E.S.; Shmarina, G.V.; Martynov, A.V.; Zakharova, N.V.; Veiko, R.V.; Umriukhin, P.E.; Kostyuk, G.P.; Kutsev, S.I.; Veiko, N.N.; Kostyuk, S.V. NADPH-oxidase 4 gene over-expression in peripheral blood lymphocytes of the schizophrenia patients. PLoS ONE 2022, 17, e0269130.
- Lubotzky, A.; Pelov, I.; Teplitz, R.; Neiman, D.; Smadja, A.; Zemmour, H.; Piyanzin, S.; Ochana, B.L.; Spalding, K.L.; Glaser, B.; et al. Elevated brain-derived cell-free DNA among patients with first psychotic episode—A proof-of-concept study. Elife 2022, 11, e76391.
- Chen, L.Y.; Qi, J.; Xu, H.L.; Lin, X.Y.; Sun, Y.J.; Ju, S.Q. The Value of Serum Cell-Free DNA Levels in Patients with Schizophrenia. Front. Psychiatry 2021, 12, 637789.
- Qi, J.; Chen, L.Y.; Shen, X.J.; Ju, S.Q. Analytical Value of Cell-Free DNA Based on Alu in Psychiatric Disorders. Front. Psychiatry 2020, 10, 992.
- Ouyang, H.; Huang, M.; Xu, Y.; Yao, Q.; Wu, X.; Zhou, D. Reduced Cell-Free Mitochondrial DNA Levels Were Induced by Antipsychotics Treatment in First-Episode Patients with Schizophrenia. Front. Psychiatry 2021, 12, 652314.
- Kageyama, Y.; Kasahara, T.; Kato, M.; Sakai, S.; Deguchi, Y.; Tani, M.; Kuroda, K.; Hattori, K.; Yoshida, S.; Goto, Y.; et al. The relationship between circulating mitochondrial DNA and inflammatory cytokines in patients with major depression. J. Affect. Disord. 2018, 233, 15–20.
- Stertz, L.; Fries, G.R.; Rosa, A.R.; Kauer-Sant’anna, M.; Ferrari, P.; Paz, A.V.; Green, C.; Cunha, Â.B.; Dal-Pizzol, F.; Gottfried, C.; et al. Damage-associated molecular patterns and immune activation in bipolar disorder. Acta Psychiatr. Scand. 2015, 132, 211–217.
- Jeong, H.; Dimick, M.K.; Sultan, A.; Duong, A.; Park, S.S.; El Soufi El Sabbagh, D.; Goldstein, B.I.; Andreazza, A.C. Peripheral biomarkers of mitochondrial dysfunction in adolescents with bipolar disorder. J. Psychiatr. Res. 2020, 123, 187–193.
- Kageyama, Y.; Deguchi, Y.; Kasahara, T.; Tani, M.; Kuroda, K.; Inoue, K.; Kato, T. Intra-individual state-dependent comparison of plasma mitochondrial DNA copy number and IL-6 levels in patients with bipolar disorder. J. Affect. Disord. 2022, 299, 644–651.
- Lindqvist, D.; Fernström, J.; Grudet, C.; Ljunggren, L.; Träskman-Bendz, L.; Ohlsson, L.; Westrin, Å. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: Associations with HPA-axis hyperactivity. Transl. Psychiatry 2016, 6, e971.
- Lindqvist, D.; Wolkowitz, O.M.; Picard, M.; Ohlsson, L.; Bersani, F.S.; Fernström, J.; Westrin, Å.; Hough, C.M.; Lin, J.; Reus, V.I.; et al. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology 2018, 43, 1557–1564.
- Fernström, J.; Ohlsson, L.; Asp, M.; Lavant, E.; Holck, A.; Grudet, C.; Westrin, Å.; Lindqvist, D. Plasma circulating cell-free mitochondrial DNA in depressive disorders. PloS ONE 2021, 16, e0259591.
- Behnke, A.; Gumpp, A.M.; Rojas, R.; Sänger, T.; Lutz-Bonengel, S.; Moser, D.; Schelling, G.; Krumbholz, A.; Kolassa, I.T. Circulating inflammatory markers, cell-free mitochondrial DNA, cortisol, endocannabinoids, and N-acylethanolamines in female depressed outpatients. World J. Biol. Psychiatry 2023, 24, 58–69.
- Meddeb, R.; Pisareva, E.; Thierry, A.R. Guidelines for the preanalytical conditions for analyzing circulating cell-free DNA. Clin. Chem. 2019, 65, 623–633.
