The clinical infections by Staphylococcus aureus caused an increase in morbidity and mortality rates and treatment costs, aggravated by the emergence of drug-resistant strains. Different nanotechnology-enabled approaches are being investigated that can improve the paradigm of therapeutics against S. aureus pathogenesis and infections. Nanomaterials provide a suitable platform to address this challenge, with the potential to control biofilm formation, intracellular parasitism and multidrug resistance where conventional therapies show limited efficacy. Herein, the large diversity of nanoparticles and their applications to combat S. aureus pathogenesis, including in combination with antibiotics and phytochemicals, is presented and their specific biological actions are highlighted.
- staphylococcus aureus
- nanotechnology
- drug delivery
- antibacterial activity
- biofilm inhibition
- Staphylococcus aureus
- nanotherapeutics
1. Introduction
The emergence of drug-resistant pathogens equipped with active defense mechanisms against different classes of antibiotics has become a global threat to human health. Conventional antibiotics belonging to various classes usually act upon bacterial pathogens by interrupting the biosynthesis of genetic materials, such as DNA, RNA and protein, cell wall/membranes, and other cellular components essential for the basic physiology of the bacteria [1]. This traditional approach has been challenged under certain conditions when the pathogens mount resistance mechanism(s) through the expression of antibiotic resistance genes, mutation of drug targets, biofilm formation, and other protective mechanisms.
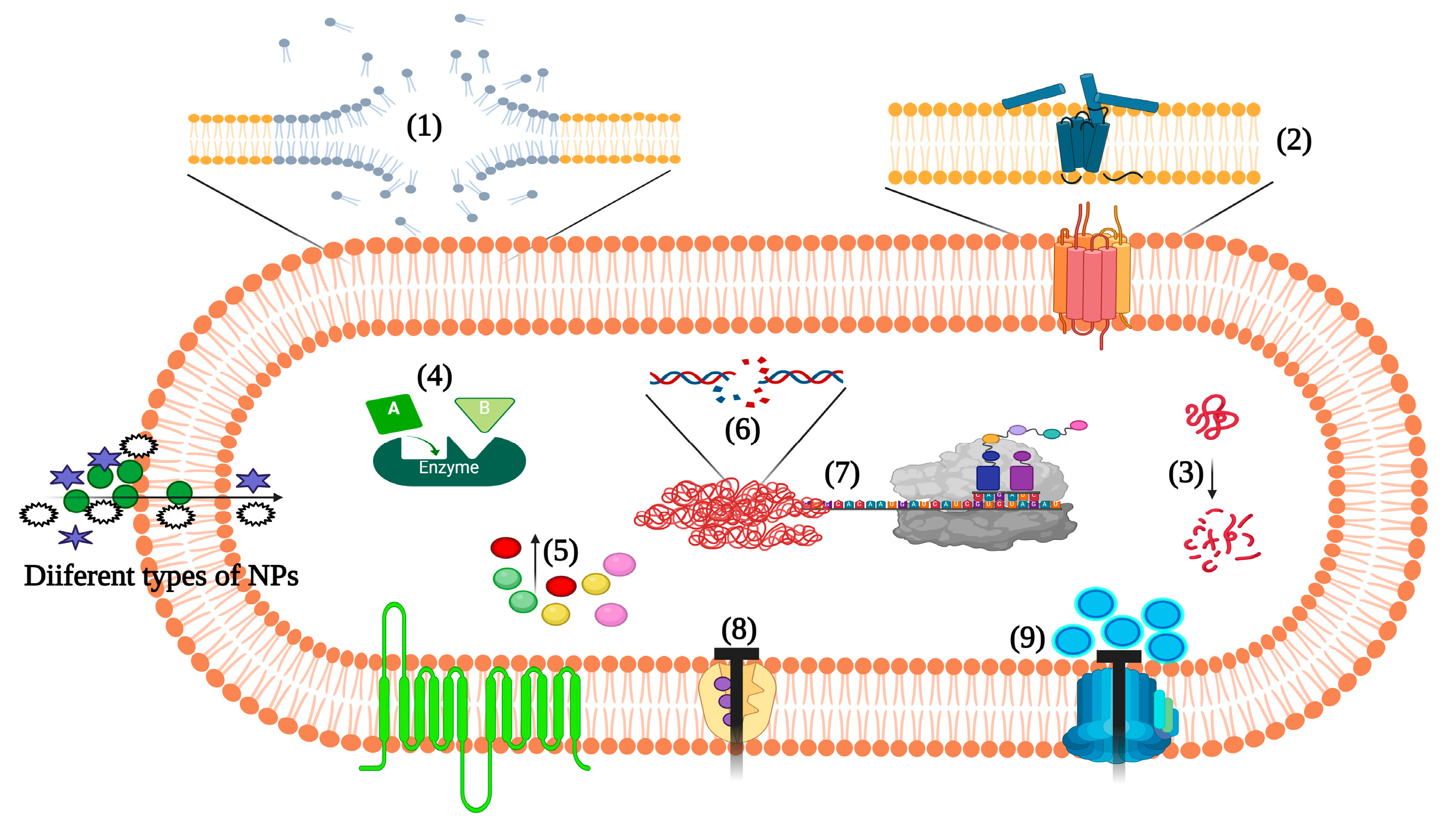
2. Nanopartechnologyicle-Based Approaches to Target Staphylococcus aureus Pathogenesis
3. Inorganic NPanoparticles
3.2.1. Gold NPanoparticles
3.2. Silver NPanoparticles
3.3. Copper NPanoparticles
3.4. Metal Oxide NPanoparticles
3.5. Action Mechanism of Metallic NPanoparticles
3.6. Silica-Based NPanoparticles
3.7. Quantum Dots and Carbon Nanodots
| Reducing or Capping Agent/Encapsulated Drug | Properties of the NPs | Biological Activities | Reference(s) |
|---|---|---|---|
| Gold NPs (GNPs) | |||
| Padina tetrastromatica-mediated synthesis of GNPs | M: Green synthesis AS: 1–20 nm S: Spherical PDI: ~23 nm |
GNPs showed an MIC of 25 µg/mL Higher concentrations of GNPs also exhibited biofilm-eradicating ability |
[77] |
| Polypeptide polymer-conjugated GNPs | M: Chemical reduction method S: Spherical AS: 23 nm AZP: 24 mV |
Polypeptide-conjugated GNPs exhibited potent antibacterial activities against clinical isolates of MDR Gram-positive bacteria, such as MRSA Excellent in vitro and in vivo biocompatibility Studies with the antioxidant N-acetyl-L-cysteine suggested that oxidative stress is responsible for the antibacterial activity of these GNPs |
[78] |
| Caffeine-loaded GNPs | S: Spherical AS: 77.9 nm |
MIC was 512 µg/mL Biofilm inhibitory and biofilm eradication concentrations of 256 and 512 µg/mL, respectively GNPs eradicated persister cells of S. aureus |
[25] |
| Silver NPs (SNPs) | |||
| Desertifilum sp.-mediated synthesis of SNPs | M: Green synthesis S: Spherical AS: 4.5–26 nm |
Comparing the growth inhibitory activity against different pathogens, MRSA was more susceptible to the SNPs (MIC 1.2 mg/mL) Anti-staphylococcal activity of SNPs was related to ROS-induced oxidative stress |
[79] |
| SNPs | M: Microwave technique S: Spherical AS: 1-3 nm AZP: Positively charged |
Interaction between SNPs and bacterial cell wall caused leakage of cytoplasmic material MIC of SNPs was 12.5 ppm against S. aureus Eradication of mixed species biofilms (Candida albicans and S. aureus) in a dose-dependent manner, with 0.53 ppm as the IC50 value SNP-functionalized catheter material was less prone to mixed species biofilm formation |
[80][81] |
| Commercial SNPs | AS: 10 nm | Photolysis of staphyloxanthin via blue light increased the anti-staphylococcal activity of SNPs Blue light reduced the MIC of SNPs from 10 µg/mL to 1 µg/mL, which is safer for mammalian cells Photolysis of staphyloxanthin increased the uptake of SNPs into the bacterial cells |
[82] |
| Piper longum mediated-synthesis of SNPs | M: Green synthesis S: Spherical AS: 10–40 nm |
SNPs were active against Bacillus cereus, S. aureus, Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, P. aeruginosa, and Salmonella typhi SNPs were active after three months of storage |
[83] |
| Gardenia thailandica leaf extract-mediated synthesis of SNPs | M: Green synthesis S: Spherical AS: 11.02–17.92 nm AZP: −6.54 ± 0.6mV |
MIC of the SNPs against S. aureus ranged from 4 to 64 µg/mL SNP at 4 × MIC and 8 × MIC eradicated the S. aureus cells at 2 h and 1 h, respectively SNPs decreased the expression of efflux pump genes norA, norB, and norC |
[84] |
| Copper (CuNPs) and copper oxide NPs (CuONPs) | |||
| Curcuma longa or Zingiber officinale extract-mediated synthesis of CuNPs | M: Green synthesis S: Spherical AS: 20–100 nm |
Agar well diffusion assay showed the antibacterial effect of CuNPs (1 and 5 mM) produced with C. longa was higher than those produced with Z. officinale | [37] |
| TH4+/CuNPs Virkon S/CuNPs Tek-Trol/CuNPs Peracetic/CuNPs DC&R/CuNPs |
AS: The ranges of particle size were 79.88–100.62 nm (TH4+), 77.74–116.49 nm (Virkon S), 82.32–115.91nm (Tek-Trol), 90.25–105.07 nm (Peracetic), and 115.15–144.86 nm (DC&R) AZP: 2.92 and 3.43 mV |
The ability of disinfectant-loaded CuNPs to eliminate the viable bacterial colonies in biofilm surfaces was studied with different concentrations and time points At a contact time of 5 min TH4+/CuNPs (1%), Tek-Trol/CuNPs (1%), DC&R/CuNPs (16%), 10 min Peracetic/CuNPs (0.5%), or 20 min Virkon S/CuNPs (2%), significantly reduced the total viable count of S. aureus |
[38] |
| CuNPs and CuONPs | M: Plasma arc discharge method AS: 78 nm (CuNPs) and 67 nm (CuONPs) |
Tested against different bacteria, CuNPs and CuONPs showed the highest zone of inhibition against S. aureus NPs induced ROS production, protein denaturation, DNA damage, and cell death |
[57] |
| CuNPs | AS: 25 nm | CuNPs showed significant anti-staphylococcal activity with reduced toxicity against fibroblasts (at 6.25 µg/mL concentration) In vivo studies using S. aureus-induced mastitis rat model indicated that CuNPs improved clinical signs faster (three days) than gentamycin (four days) CuNPs reversed the S. aureus-induced histopathological changes in the mammary gland and, on the 5th day after treatment, bacterial load, mammary gland weight, and oxidative stress parameters were lower compared to the disease control and antibiotic-treated animals |
[85] |
| Other metallic NPs | |||
| Lactobacillus plantarum TA4-mediated synthesis of ZnONPs | S: Oval AS: 29.7 nm |
ZnONPs were effective against S. aureus from poultry samples (disc diffusion assay) MIC and MBC values were 30 and 100 µg/mL, respectively ZnONPs inhibited biofilm formation in a dose-dependent manner The results suggested that ROS generation was the underlying antibacterial mechanism |
[86] |
| Pancreatin-doped ZnONPs | M: Precipitation method S: Hexagonal AS: 85 nm |
Antibacterial and virulence inhibitory activity against MRSA Protein leakage and generation of ROS were possible antibacterial mechanisms Pancreatin-doped ZnONPs sensitized the cells to vancomycin |
[87] |
| Rhamnolipid-coated ZnONPs | AS: From 40 to 55 nm S: Spherical |
NPs at 0.5 mg/mL had low toxicity to fibroblast cells and low hemolytic activity NPs treatment reduced the bacterial burden in infected wound in rats, revealing a rapid wound healing within five days compared to the rhamnolipid- and clindamycin-treated wounds In histopathological analysis, the NP-treated animals showed rapid remodeling of the epidermis and the presence of ample amounts of dermal cells on the 5th day of treatment |
[88][89] |
| Aspergillus terreus S1 mediated-synthesis of MgONPs | M: Green synthesis S: Spherical AS: 8–38 nm PDI: 0.2 |
Growth inhibitory activity (MIC 200 μg/mL) against B. subtilis (13.3 ± 1.9 mm, inhibition zone), E. coli (11.3 ± 0.6 mm), C. albicans (12.8 ± 0.3 mm), P. aeruginosa (14.7 ± 1.9 mm), and S. aureus (11.3 ± 0.6 mm) | [90] |
| Carum copticum extract-mediated synthesis of TiONPs | M: Green synthesis S: Spherical or spheroid shaped AS: ~12 nm |
Inhibition of EPS secretion and rupture of preformed biofilms of S. aureus | [91] |
| Ochradenus arabicus leaf extract-mediated synthesis of TiONPs | M: Green synthesis AS: 26.48 nm |
MIC of the TiONPs was 32 µg/mL TiONPs at 0.5 × MIC inhibited biofilm formation and EPS production by MRSA to approx. 50% MRSA strains increased production of ROS upon treatment with the TiONPs |
[92] |
| Mesoporous Silica NPs (MSNs) | |||
| Enzyme-functionalized MSN | M: Stober method S: Spherical AS: Lys@MSN (38 ± 5 nm), Ser@MSN (31 ± 7 nm), and DN@MSN (35 ± 4 nm) AZP: Lys@MSN (+12 ± 5 mV), Ser@MSN (−22 ± 5 mV), and DN@MSN (+27 ± 5 mV) |
Enzymes lysostaphin (Lys@MSN), serrapeptase (Ser@MSN), and DNase I (DN@MSN) were immobilized in MSNs Lys@MSNs targeted MRSA and MSSA growth by inducing cell lysis The other two enzymes immobilized in MSNs targeted the biofilm formation of S. aureus by hampering the production of proteins and eDNA Lys@MSNs showed a 7.5- and 5-fold decrease in MIC and MBIC values compared to free lysostaphin |
[93] |
| Rifampicin-loaded MSN | M: Solvent extraction (e) and calcination (c) methods AS: 40 nm (c & e), 80 nm (c) AZP: 15 (40e), 13 (40c), and 14 mV (80c) DL: 29 (40e), 33 (40c), and 38% (80c) |
Hydrophilic e-MSN particles (prepared using solvent extraction) demonstrated a > 2-fold increase in Caco-2 cell uptake MSNs were efficacious against small colony variant S. aureus hosted within Caco-2 cells Compared to free rifampicin, the MSNs loaded with rifampicin reduced the level of S. aureus in Caco-2 cells 2.5-fold |
[61] |
| Moxifloxacin/rifampicin-loaded MSN (gelatine/colistin coated) | M: Stober method AS: 396 nm AZP: -29.2 ± 0.65 mV |
Antibiotic-loaded MSNs were studied against MRSA osteomyelitis both in vitro and in vivo MIC of the moxifloxacin and rifampicin MSNs were 3.906 and 0.977 µg/mL, respectively Intraosseous injection of MSNs decorated with aspartic acid hexapeptide (D6, affinity towards bone tissue) reduced S. aureus load to 92% in infected rabbit femurs within 24 h MSNs showed no toxicity towards osteoblasts and macrophages in vitro, but some effects on osteoclasts over time (72 h) NPs reduced biofilm formation and the expression of the proteases staphopain, SplF, and V8 protease, whereas they increased the expression of aureolysin and the transcriptional regulator protein Rot |
[94] |
| Vancomycin-loaded MSN | M: Impregnation approach S: Spherical AS: 100 nm AZP: +26.5 mV |
Antibiotic-loaded MSNs targeting bone and MRSA presented an MIC of 16 µg/mL Compared to treatment with free vancomycin, the targeted MSNs improved the recovery from orthopedic implant-related infections with MRSA in rats Hemolytic and studies with bone marrow mesenchymal stem cells indicated the biocompatibility of the MSNs, and no abnormalities were observed in the heart, spleen, liver, lung, or kidneys of treated rats |
[95] |
| Quantum dots (QDs) and Carbon nanodots (CND) | |||
| p-Coumaric acid QDs | M: Wet milling approach AS: 8.9 ± 3.7 nm AZP: −3.73 mV |
Antimicrobial activity against a wide spectrum of foodborne microorganisms At minimal lethal concentration (250 µg/mL), 99.9% killing of bacterial cells was observed throughout the experiment time |
[96] |
| Carbon QDs from gentamycin sulfate | M: Calcination method (180 °C optimal temperature) S: Spherical AS: 2–8 nm AZP: 10.9 mV |
QDs effectively cleared bacterial pathogens like E. coli and S. aureus (MIC was 1.59 and 50.8 ng/mL at pH 5.5 and 7.4, respectively) QDs at 80 µg/mL eradicated (90%) preformed biofilms, whereas the gentamycin sulfate at the same concentration reduced only 10% of the biofilms QDs showed a low toxic profile against mammalian 3T3 cells, even at 2 mg/mL concentration |
[97] |
| Carbon dots from m-aminophenol and tartaric acid | M: Hydrothermal method S: Spherical AS: 5–9 nm AZP: +33.2 ± 0.99 mV |
The positively charged carbon dots showed anti-staphylococcal activity and low toxicity toward HeLa cells The carbon dots were selectively absorbed on the cell surface through electrostatic interactions |
[98] |
| Carbon dots from levofloxacin hydrochloride | S: Spherical AS: 1.25 nm |
MIC of the carbon dots against S. aureus was 128 µg/mL Mechanisms of electrostatic interaction for surface adherence and bacterial cell wall disruption were implicated in the antibacterial action No cytotoxicity was observed towards 293T cells (viability greater than 80% at a concentration of 100 μg/mL) |
[99] |
| Negatively charged CNDs | M: Microwave-assisted synthesis AS: 2.5 nm AZP: −11.06 mV |
Inhibitory activity against MRSA and vancomycin-intermediate S. aureus (MIC of 630 μg/mL) | [100] |
| CNDs from curcumin and citric acid | M: Hydrothermal method AZD: −15.1 mV |
CNDs showed a broad range of antimicrobial and antibiofilm activity Bactericidal efficiency was maximal at 375 μg/mL against S. aureus, E. coli, P. aeruginosa, and B. subtilis |
[76] |
4. Organic NPanoparticles
4.1. Lipid-Based NPanoparticles
Liposomes
Niosomes
Quatsomes
Micelles
Stimulated Phase-Shift Acoustic Nanodroplets/Nanobubbles
Solid-Lipid NPanoparticles
4.2. Polymer Based NPanoparticles
Polymeric NPanoparticles
Dendrimers
Cyclodextrins
| Reducing or Capping Agent/ Encapsulated Drug |
Properties of the NPs | Biological Activities | Reference |
|---|---|---|---|
| Liposomes | |||
| Lecithin and Tween-80 liposomes with Laurus nobilis leaf extract | M: Ultrasound AS: 99.05 ± 2.98 nm EE: 73.76 ± 1.10% |
MIC and MBC of plant extracts were between 100 and 500 ppm At 1500 ppm, the loaded liposomes inhibited oxidation, bacterial growth, and spoilage of minced beef inoculated with E. coli and S. aureus |
[165] |
| Lecithin liposomes with co-encapsulated berberine and curcumin | M: Film hydration AS: 253 ± 22 nm AZP: −57 ± 4 mV EE: 57 ± 3% |
MIC of free berberine and curcumin were 62 and 250 µg/mL, respectively Encapsulation reduced the MIC of the drugs by approximately half and more efficiently prevented MRSA biofilm formation Free berberine and curcumin combinations showed an MIC of 31/16 µg/mL with an FIC index of 0.56 (no interaction), while the dual drug-loaded liposomes showed an MIC of 8/10 µg/mL with an FIC index of 0.13 (synergy) The liposomes were more efficient than clindamycin in reducing intracellular infection |
[166] |
| Niosomes | |||
| Ciprofloxacin-loaded niosomes | M: Remote-loading technique S: Spherical AS: 123 nm PDI: 0.198 EE: 79.25% |
Stable ciprofloxacin-loaded niosomes showed MIC in the range of 2–4 µg/mL against the S. aureus strains, a 4- to 5-fold increase in antibacterial potency compared to the free drug Sub-MIC inhibited the biofilm formation of ciprofloxacin-resistant S. aureus and down-regulated the icaB gene |
[121] |
| Cefazolin-loaded niosomes | M: Film hydration S: Spherical AS: 100 nm AZP: −63 mV |
Cefazolin-containing niosomes removed one- to five-day-old biofilms in a concentration-dependent manner (MRSA isolates from patients with pressure sores and diabetic ulcers) Histopathological results indicated that mice treated with cefazolin-loaded niosomes recovered faster than those treated with the free drug or the untreated group |
[167] |
| Quatsomes | |||
| Vancomycin-loaded quatsomes from quaternary bicephalic surfactants and cholesterol | M: Sonication/dispersion method AS: 123 nm AZP: 0.169 mV EE: 52.2% |
The pH-responsive quatsomes showed 32- and 8-fold lower MICs against MRSA at pH 6 and 7.4, respectively, compared to the free vancomycin The drug-loaded quatsomes caused more significant membrane damage, had a bactericidal effect, and counteracted MRSA biofilms in vitro In a mouse skin infection model, the quatsome formulation performed better than the free antibiotic |
[124] |
| Cetylpyridinium chloride (CPC)-quatsomes | ND | No toxicity towards human airway epithelial (NuLi-1) cells Low concentration inhibited the planktonic and biofilm cells of S. aureus and P. aeruginosa |
[125] |
| Micelles | |||
| Platensimycin-loaded micelles constructed using [poly(lactic-co-glycolic acid)-poly(2-ethyl-2-oxazoline) (PLGA−PEOz)] and PLGA-poly(ethylene glycol) (PLGA-PEG) | AS: 183 nm (PLGA−PEOz), 195 nm (PLGA-PEG) AZP: -5.37 (PLGA−PEOz), −5.42 (PLGA-PEG) EE: 41.7% (PLGA−PEOz), 40.4% (PLGA-PEG) |
Improved results against intracellular MRSA in a macrophage infection model Compared to the free drug, drug-loaded micelles showed higher potential against MRSA-induced peritonitis in mice (dose 20 mg/kg, increased survival and reduced colonization) The drug-loaded micelles were not toxic to the cells nor the animals Cmax after i.p. injection of the free drug was 28 ± 9 μg/mL, but concentrations greater than 50 μg/mL were measured after administering the encapsulated drug |
[129] |
| Solid Lipid NPs (SLNs) | |||
| Curcumin-loaded SLNs | M: Microemulsion method S: Spherical AS: 126.87 ± 0.94 nm PDI: 0.21 ZP: 30 ± 0.3 mV EE: 99.96% DL: 1.8% |
Curcumin SLNs were effective against pathogens such as S. aureus and E. coli Lower MIC value (142 μg/mL) than free curcumin (1000 μg/mL) The curcumin SLNs reduced the pathogens’ cell counts in contaminated food for eight days |
[168] |
| Anacardic acid encapsulated in SLNs | M: Hot homogenization S: Spherical AS: 203.6 ± 3.05 nm PDI: 0.277 ± 0.02 ZP: −21.4 ± 2.81 mV DL: 76.4 ± 1.9% |
Stable for 90 days and non-toxic to the human keratinocyte cell line HaCat High anti-staphylococcal and biofilm inhibitory activities |
[133] |
| Polymeric NPs | |||
| Rifampicin-loaded poly-lactic acid NPs | M: Nanoprecipitation S: Spherical AS: 144 nm PDI: 0.08 AZP: −56 ± 5 mV DL: 2.2% EE: 90.5% |
NPs coated with poly-lysine were more active against the growth and biofilms of S. aureus, presumably due to enhanced interaction and slow penetration into S. aureus biofilms | [169] |
| Citrus reticulata essential oil loaded in chitosan NPs | AS: 131–162 nm EE: 67.32%–82.35% AZP: 30 mV |
The loaded NPs disturbed bacterial cell membranes and displayed high anti-staphylococcal activity, as well as inhibition of biofilm formation and premature biofilms of S. aureus | [170] |
| Chitosan functionalized SNP by Sygyzium aromaticum | M: Biogenic synthesis S: Spherical AS: 30–40 nm |
Effective against MRSA and VRSA Lethal toxicity towards HeLa cells and brine shrimp was observed at 325 μg/mL, which is three times higher than the effective concentration showing anticoagulation, antiplatelet, and thrombolytic activities |
[171] |
| Dendrimers | |||
| Platensimycin-loaded PLGA and PAMAM dendrimer NPs | M: Emulsification-evaporation AS: 175.6 nm (PLGA) and 218.1 nm (PAMAM) PDI: 0.10 (PLGA) and 0.17 (PAMAM) AZP: −17.7 mV (PLGA) and 17.2 mV (PAMAM) DL: 7.81% (PLGA) and 8.42% (PAMAM) EE: 62.1% (PLGA) and 63.2% (PAMAM) |
Inhibited MRSA growth and biofilms and killed the bacteria in a macrophage cell model more efficiently than the free drug Treatment with both types of drug-loaded NPs was effective against MRSA peritoneal infection in the mice models, with reduction of MRSA in the blood and kidneys, and full survival for 7 days, while the animals treated with the same dose of free drug (10 mg/kg, i.p.) died in 24 h In pharmacokinetic study in rats, the NPs formulations provided a 2- to 4-fold higher AUC and extended the mean residence time of the drug (Cmax approx. 80 μg/mL) Loaded PLGA and PAMAM NPs showed no appreciable effect on RAW 264.7 cell viability at concentrations well above those providing antibacterial activity (below 100 μg/mL) |
[151] |
| PAMAM dendrimers with amide-conjugated vancomycin and incorporated SNP | M: Drug-PAMAM with amide conjugation AS: Dual drug-conjugated dendrimers with 68 nm AZP: 27.5 mV |
5–7-log reduction in colony-forming units of VRSA Antimicrobial resistance induction was not detected in a susceptible strain, in contrast to using the free antibiotic Good biocompatibility with IH 3T3 fibroblasts and HUVEC cells (up to 8 µg SNP/mL) and low hemolytic effects Irrigation of infected wounds in mice with the dual-drug dendrimers cleared VRSA and reduced the accumulation of granulocytes at the wound site more efficiently than the free antibiotic or the SNP-only PAMAM dendrimers |
[153] |
| Polyurea (PURE) oligoethyleneimine (OEI) dendrimers | M: Grafting oligo-(2-ethyl-oxazoline) in polyurea dendrimer, followed by acid hydrolysis AZP: cationic Mw 82,871 g/mol (PURE-G4-OEI-48) and 160,788 g/mol (PURE-G3-OEI-24) |
MIC and MBC against MRSA, MSSA, Streptococcus pneumonia, Gram-negative bacteria and Candida strains below 10 μM (lower than 1 μM in the case of MRSA) PURE-G4-OEI-48 effective against Pseudomonas aeruginosa and MRSA infections in a Galleria mellonella insect model Up to 6 μM, no toxicity was observed against human bronchial epithelial 16HBE14o- and vaginal VK2 (E6/E7) cell lines, nor an effect on the health index scores of G. mellonella Live/dead assays, SEM, and molecular dynamic simulations supported a fast-killing mechanism via membrane disruption |
[158] |
References
- Wright, G.D. Mechanisms of resistance to antibiotics. Curr. Opin. Chem. Biol. 2003, 7, 563–569.
- Römling, U.; Kjelleberg, S.; Normark, S.; Nyman, L.; Uhlin, B.E.; Åkerlund, B. Microbial biofilm formation: A need to act. J. Intern. Med. 2014, 276, 98–110.
- Alonso, B.; Pérez-Granda, M.J.; Latorre, M.C.; Sánchez-Carrillo, C.; Bouza, E.; Muñoz, P.; Guembe, M. Production of biofilm by Staphylococcus aureus: Association with infective endocarditis? Enferm. Infecc. Y Microbiol. Clín. 2021, 40, 418–422.
- Gittins, D.I.; Bethell, D.; Schiffrin, D.J.; Nichols, R.J. A nanometre-scale electronic switch consisting of a metal cluster and redox-addressable groups. Nature 2000, 408, 67–69.
- Sathishkumar, M.; Sneha, K.; Yun, Y.-S. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour. Technol. 2010, 101, 7958–7965.
- Elegbede, J.A.; Lateef, A. Green synthesis of silver (Ag), gold (Au), and silver–gold (Ag–Au) alloy nanoparticles: A review on recent advances, trends, and biomedical applications. In Nanotechnology and Nanomaterial Applications in Food, Health, and Biomedical Sciences; Apple Academic Press: Palm Bay, FI, USA, 2019; pp. 3–89.
- Lateef, A.; Ojo, S.A.; Elegbede, J.A.; Akinola, P.O.; Akanni, E.O. Nanomedical applications of nanoparticles for blood coagulation disorders. Environ. Nanotechnol. 2018, 14, 243–277.
- Pinto, R.M.; Lopes-de-Campos, D.; Martins, M.C.L.; Van Dijck, P.; Nunes, C.; Reis, S. Impact of nanosystems in Staphylococcus aureus biofilms treatment. FEMS Microbiol. Rev. 2019, 43, 622–641.
- Alavi, M.; Rai, M. Recent advances in antibacterial applications of metal nanoparticles (MNPs) and metal nanocomposites (MNCs) against multidrug-resistant (MDR) bacteria. Expert Rev. Anti-Infect. Ther. 2019, 17, 419–428.
- Boswell, G.W.; Buell, D.; Bekersky, I. AmBisome (Liposomal Amphotericin B): A Comparative Review. J. Clin. Pharmacol. 1998, 38, 583–592.
- Gupta, N.; Rai, D.B.; Jangid, A.K.; Kulhari, H. Chapter 7—Use of nanotechnology in antimicrobial therapy. In Methods in Microbiology; Gurtler, V., Ball, A.S., Soni, S., Eds.; Academic Press: Cambridge, UK, 2019; Volume 46, pp. 143–172.
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384.
- Salah, R.; Karmy, M.; Abdelraouf, A.; Kotb, S. Evaluation of the bactericidal effect of silver nanoparticles against methicillin resistant Staphylococcus aureus (MRSA) and methicillin sensitive Staphylococcus aureus (MSSA) strains isolated from mastitic milk of small ruminants and their surrounding environment in Aswan, Egypt. J. Vet. Med. Res. 2021, 27, 143–151.
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–biofilm interactions: The role of the EPS matrix. Trends Microbiol. 2019, 27, 915–926.
- Singh, B.; Vuddanda, P.R.; Vijayakumar, M.; Kumar, V.; Saxena, P.S.; Singh, S. Cefuroxime axetil loaded solid lipid nanoparticles for enhanced activity against S. Aureus Biofilm. Colloids Surf. B Biointerfaces 2014, 121, 92–98.
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382.
- Kankala, R.K.; Wang, S.B.; Chen, A.Z. Nanoarchitecting Hierarchical Mesoporous Siliceous Frameworks: A New Way Forward. Iscience 2020, 23, 101687.
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules 2021, 26, 844.
- De Souza, C.D.; Nogueira, B.R.; Rostelato, M.E.C. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740.
- Kherde, Y.; Aguilar, Z.P.; Zystein, L.; Rodgers, H.M.; Hamilton, W. Gold nanoparticles: Various methods of synthesis and antibacterial applications. Front. Biosci. 2014, 19, 1320–1344.
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold nanoparticles: An efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials 2016, 6, 71.
- Dasari, T.S.; Zhang, Y.; Yu, H. Antibacterial activity and cytotoxicity of gold (I) and (III) ions and gold nanoparticles. Biochem. Pharmacol. 2015, 4, 199.
- Boda, S.K.; Broda, J.; Schiefer, F.; Weber-Heynemann, J.; Hoss, M.; Simon, U.; Basu, B.; Jahnen-Dechent, W. Cytotoxicity of ultrasmall gold nanoparticles on planktonic and biofilm encapsulated Gram-positive staphylococci. Small 2015, 11, 3183–3193.
- Gouyau, J.; Duval, R.E.; Boudier, A.; Lamouroux, E. Investigation of nanoparticle metallic core antibacterial activity: Gold and silver nanoparticles against Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 1905.
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; El-Agamy Farh, M.; Yang, D.C. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 811–816.
- Khan, F.; Park, S.-K.; Bamunuarachchi, N.I.; Oh, D.; Kim, Y.-M. Caffeine-loaded gold nanoparticles: Antibiofilm and anti-persister activities against pathogenic bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 3717–3731.
- Bar, H. One pot green synthesis of gold nanoparticles using Piper betle leaf extract and their antibacterial activities. Adv. Mater. Res. 2021, 1163, 106–116.
- Hameed, S.; Wang, Y.; Zhao, L.; Xie, L.; Ying, Y. Shape-dependent significant physical mutilation and antibacterial mechanisms of gold nanoparticles against foodborne bacterial pathogens (Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus) at lower concentrations. Mater. Sci. Eng. C 2020, 108, 110338.
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468.
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837.
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828.
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874.
- Hussein, E.A.M.; Mohammad, A.A.-H.; Harraz, F.A.; Ahsan, M.F. Biologically synthesized silver nanoparticles for enhancing tetracycline activity against Staphylococcus aureus and Klebsiella pneumoniae. Braz. Arch. Biol. Technol. 2019, 62.
- Goswami, S.; Sahareen, T.; Singh, M.; Kumar, S. Role of biogenic silver nanoparticles in disruption of cell–cell adhesion in Staphylococcus aureus and Escherichia coli biofilm. J. Ind. Eng. Chem. 2015, 26, 73–80.
- Zautner, A.E.; Krause, M.; Stropahl, G.; Holtfreter, S.; Frickmann, H.; Maletzki, C.; Kreikemeyer, B.; Pau, H.W.; Podbielski, A. Intracellular persisting Staphylococcus aureus is the major pathogen in recurrent tonsillitis. PLoS ONE 2010, 5, e9452.
- Kang, J.; Dietz, M.J.; Hughes, K.; Xing, M.; Li, B. Silver nanoparticles present high intracellular and extracellular killing against Staphylococcus aureus. J. Antimicrob. Chemother. 2019, 74, 1578–1585.
- Parveen, F.; Sannakki, B.; Mandke, M.V.; Pathan, H.M. Copper nanoparticles: Synthesis methods and its light harvesting performance. Sol. Energy Mater. Sol. Cells 2016, 144, 371–382.
- Varghese, B.; Kurian, M.; Krishna, S.; Athira, T. Biochemical synthesis of copper nanoparticles using Zingiber officinalis and Curcuma longa: Characterization and antibacterial activity study. Mater. Today Proc. 2020, 25, 302–306.
- Elsayed, M.M.; Elgohary, F.A.; Zakaria, A.I.; Elkenany, R.M.; El-Khateeb, A.Y. Novel eradication methods for Staphylococcus aureus biofilm in poultry farms and abattoirs using disinfectants loaded onto silver and copper nanoparticles. Environ. Sci. Pollut. Res. 2020, 27, 30716–30728.
- Chen, J.; Mao, S.; Xu, Z.; Ding, W. Various antibacterial mechanisms of biosynthesized copper oxide nanoparticles against soilborne Ralstonia solanacearum. RSC Adv. 2019, 9, 3788–3799.
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int. J. Mol. Sci. 2021, 22, 4595.
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4927–4939.
- Liu, Y.; Li, Y.; Deng, L.; Zou, L.; Feng, F.; Zhang, H. Hydrophobic Ethylcellulose/Gelatin Nanofibers Containing Zinc Oxide Nanoparticles for Antimicrobial Packaging. J. Agric. Food Chem. 2018, 66, 9498–9506.
- Kim, M.-H.; Yamayoshi, I.; Mathew, S.; Lin, H.; Nayfach, J.; Simon, S.I. Magnetic nanoparticle targeted hyperthermia of cutaneous Staphylococcus aureus infection. Ann. Biomed. Eng. 2013, 41, 598–609.
- Fang, C.-H.; Tsai, P.-I.; Huang, S.-W.; Sun, J.-S.; Chang, J.Z.-C.; Shen, H.-H.; Chen, S.-Y.; Lin, F.H.; Hsu, L.-T.; Chen, Y.-C. Magnetic hyperthermia enhance the treatment efficacy of peri-implant osteomyelitis. BMC Infect. Dis. 2017, 17, 516.
- Aureliano, M.; Marques-da-Silva, D.; Serrano, A.; Martins, J.; Faleiro, L.; Fonseca, C.; Fraqueza, G.; Lagoa, R. Polyoxometalates with anticancer, antibacterial and antiviral activities. In Polyoxometalates: Advances, Properties, and Applications; Rubio, L., Vilela, J., Artetxe, B., Gutiérrez-Zorrilla, J., Eds.; Jenny Stanford Publishing: United Square, Singapore, 2022; pp. 309–358.
- Ekielski, A. Interactions Between Food Ingredients and Nanocomponents Used for Composite Packaging. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 669–674.
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium oxide nanoparticles: Effective agricultural antibacterial agent against Ralstonia Solanacearum. Front. Microbiol. 2018, 9, 790.
- Akhtar, S.; Shahzad, K.; Mushtaq, S.; Ali, I.; Rafe, M.H.; Fazal-ul-Karim, S.M. Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater. Res. Express 2019, 6, 105409.
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganisms 2020, 8, 1545.
- Gao, W.; Thamphiwatana, S.; Angsantikul, P.; Zhang, L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 532–547.
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227.
- Zaidi, S.; Misba, L.; Khan, A.U. Nano-therapeutics: A revolution in infection control in post antibiotic era. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2281–2301.
- Niemirowicz, K.; Swiecicka, I.; Wilczewska, A.Z.; Misztalewska, I.; Kalska-Szostko, B.; Bienias, K.; Bucki, R.; Car, H. Gold-functionalized magnetic nanoparticles restrict growth of Pseudomonas aeruginosa. Int. J. Nanomed. 2014, 9, 2217.
- Su, Y.; Zheng, X.; Chen, Y.; Li, M.; Liu, K. Alteration of intracellular protein expressions as a key mechanism of the deterioration of bacterial denitrification caused by copper oxide nanoparticles. Sci. Rep. 2015, 5, 15824.
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178.
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444.
- Tharchanaa, S.; Priyanka, K.; Preethi, K.; Shanmugavelayutham, G. Facile synthesis of Cu and CuO nanoparticles from copper scrap using plasma arc discharge method and evaluation of antibacterial activity. Mater. Technol. 2021, 36, 97–104.
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124.
- Gounani, Z.; Asadollahi, M.A.; Pedersen, J.N.; Lyngsø, J.; Pedersen, J.S.; Arpanaei, A.; Meyer, R.L. Mesoporous silica nanoparticles carrying multiple antibiotics provide enhanced synergistic effect and improved biocompatibility. Colloids Surf. B Biointerfaces 2019, 175, 498–508.
- Slomberg, D.L.; Lu, Y.; Broadnax, A.D.; Hunter, R.A.; Carpenter, A.W.; Schoenfisch, M.H. Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 9322–9329.
- Joyce, P.; Ulmefors, H.; Maghrebi, S.; Subramaniam, S.; Wignall, A.; Jõemetsa, S.; Höök, F.; Prestidge, C.A. Enhancing the cellular uptake and antibacterial activity of rifampicin through encapsulation in mesoporous silica nanoparticles. Nanomaterials 2020, 10, 815.
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.-G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018, 2, 95–103.
- Wu, C.; Zhu, Y.; Wu, T.; Wang, L.; Yuan, Y.; Chen, J.; Hu, Y.; Pang, J. Enhanced functional properties of biopolymer film incorporated with curcurmin-loaded mesoporous silica nanoparticles for food packaging. Food Chem. 2019, 288, 139–145.
- Tamanna, T.; Landersdorfer, C.B.; Ng, H.J.; Bulitta, J.B.; Wood, P.; Yu, A. Prolonged and continuous antibacterial and anti-biofilm activities of thin films embedded with gentamicin-loaded mesoporous silica nanoparticles. Appl. Nanosci. 2018, 8, 1471–1482.
- Vijayan, V.; Uthaman, S.; Park, I.-K. Cell membrane coated nanoparticles: An emerging biomimetic nanoplatform for targeted bioimaging and therapy. Biomim. Med. Mater. 2018, 1064, 45–59.
- Gao, W.; Zhang, L. Coating nanoparticles with cell membranes for targeted drug delivery. J. Drug Target. 2015, 23, 619–626.
- Yang, S.; Han, X.; Yang, Y.; Qiao, H.; Yu, Z.; Liu, Y.; Wang, J.; Tang, T. Bacteria-targeting nanoparticles with microenvironment-responsive antibiotic release to eliminate intracellular Staphylococcus aureus and associated infection. ACS Appl. Mater. Interfaces 2018, 10, 14299–14311.
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Haselmann, G.M.; Wang, J.; Eder, D.; Schneider-Stickler, B. Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix. Carbohydr. Polym. 2018, 200, 35–42.
- Alavi, M.; Jabari, E.; Jabbari, E. Functionalized carbon-based nanomaterials and quantum dots with antibacterial activity: A review. Expert Rev. Anti-Infect. Ther. 2021, 19, 35–44.
- Singh, A.K.; Prakash, P.; Singh, R.; Nandy, N.; Firdaus, Z.; Bansal, M.; Singh, R.K.; Srivastava, A.; Roy, J.K.; Mishra, B. Curcumin quantum dots mediated degradation of bacterial biofilms. Front. Microbiol. 2017, 8, 1517.
- Meikhail, M.; Abdelghany, A.; Awad, W. Role of CdSe quantum dots in the structure and antibacterial activity of chitosan/poly ε-caprolactone thin films. Egypt. J. Basic Appl. Sci. 2018, 5, 138–144.
- Wansapura, P.T.; Dassanayake, R.S.; Hamood, A.; Tran, P.; Moussa, H.; Abidi, N. Preparation of chitin-CdTe quantum dots films and antibacterial effect on Staphylococcus aureus and Pseudomonas aeruginosa. J. Appl. Polym. Sci. 2017, 134.
- Openda, Y.I.; Sen, P.; Managa, M.; Nyokong, T. Acetophenone substituted phthalocyanines and their graphene quantum dots conjugates as photosensitizers for photodynamic antimicrobial chemotherapy against Staphylococcus aureus. Photodiagnosis Photodyn. Ther. 2020, 29, 101607.
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737.
- Saravanan, A.; Maruthapandi, M.; Das, P.; Luong, J.H.; Gedanken, A. Green synthesis of multifunctional carbon dots with antibacterial activities. Nanomaterials 2021, 11, 369.
- Lu, F.; Ma, Y.; Wang, H.; Zhang, M.; Wang, B.; Zhang, Y.; Huang, H.; Liao, F.; Liu, Y.; Kang, Z. Water-solvable carbon dots derived from curcumin and citric acid with enhanced broad-spectrum antibacterial and antibiofilm activity. Mater. Today Commun. 2021, 26, 102000.
- Salam, F.D.; Vinita, M.N.; Puja, P.; Prakash, S.; Yuvakkumar, R.; Kumar, P. Anti-bacterial and anti-biofilm efficacies of bioinspired gold nanoparticles. Mater. Lett. 2020, 261, 126998.
- Zhang, W.; Wu, Y.; Liu, L.; Xiao, X.; Cong, Z.; Shao, N.; Qiao, Z.; Chen, K.; Liu, S.; Zhang, H.; et al. The membrane-targeting mechanism of host defense peptides inspiring the design of polypeptide-conjugated gold nanoparticles exhibiting effective antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Mater. Chem. B 2021, 9, 5092–5101.
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel biogenic silver nanoparticle-induced reactive oxygen species inhibit the biofilm formation and virulence activities of methicillin-resistant Staphylococcus aureus (MRSA) strain. Front. Bioeng. Biotechnol. 2020, 8, 433.
- Lara, H.H.; Lopez-Ribot, J.L. Inhibition of mixed biofilms of Candida albicans and methicillin-resistant Staphylococcus aureus by positively charged silver nanoparticles and functionalized silicone elastomers. Pathogens 2020, 9, 784.
- Romero-Urbina, D.G.; Lara, H.H.; Velázquez-Salazar, J.J.; Arellano-Jiménez, M.J.; Larios, E.; Srinivasan, A.; Lopez-Ribot, J.L.; Yacamán, M.J. Ultrastructural changes in methicillin-resistant Staphylococcus aureus induced by positively charged silver nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2396–2405.
- Jusuf, S.; Hui, J.; Dong, P.-T.; Cheng, J.-X. Staphyloxanthin photolysis potentiates low concentration silver nanoparticles in eradication of methicillin-resistant Staphylococcus aureus. J. Phys. Chem. C 2020, 124, 5321–5330.
- Huang, H.; Shan, K.; Liu, J.; Tao, X.; Periyasamy, S.; Durairaj, S.; Jiang, Z.; Jacob, J.A. Synthesis, optimization and characterization of silver nanoparticles using the catkin extract of Piper longum for bactericidal effect against food-borne pathogens via conventional and mathematical approaches. Bioorg. Chem. 2020, 103, 104230.
- Attallah, N.G.M.; Elekhnawy, E.; Negm, W.A.; Hussein, I.A.; Mokhtar, F.A.; Al-Fakhrany, O.M. In vivo and in vitro antimicrobial activity of biogenic silver nanoparticles against Staphylococcus aureus clinical isolates. Pharmaceuticals 2022, 15, 194.
- Taifa, S.; Muhee, A.; Bhat, R.A.; Nabi, S.U.I.; Roy, A.; Rather, G.A.; Khan, A.A.; Bashir, S.M.; Patwekar, M.; Wahab, S.; et al. Evaluation of therapeutic efficacy of copper nanoparticles in Staphylococcus aureus-induced rat mastitis model. J. Nanomater. 2022, 2022, 7124114.
- Mohd Yusof, H.; Abdul Rahman, N.A.; Mohamad, R.; Hasanah Zaidan, U.; Samsudin, A.A. Antibacterial potential of biosynthesized zinc oxide nanoparticles against poultry-associated foodborne pathogens: An in vitro study. Animals 2021, 11, 2093.
- Banerjee, S.; Vishakha, K.; Das, S.; Dutta, M.; Mukherjee, D.; Mondal, J.; Mondal, S.; Ganguli, A. Antibacterial, anti-biofilm activity and mechanism of action of pancreatin doped zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus. Colloids Surf. B Biointerfaces 2020, 190, 110921.
- Malakar, C.; Kashyap, B.; Kalita, M.C.; Deka, S. Wound healing efficacy of rhamnolipid-coated zinc oxide nanoparticle along with its in vivo antibacterial efficacy against Staphylococcus aureus. Exp. Dermatol. 2022.
- Malakar, C.; Patowary, K.; Deka, S.; Kalita, M.C. Synthesis, characterization, and evaluation of antibacterial efficacy of rhamnolipid-coated zinc oxide nanoparticles against Staphylococcus aureus. World J. Microbiol. Biotechnol. 2021, 37, 193.
- Saied, E.; Eid, A.M.; Hassan, S.E.-D.; Salem, S.S.; Radwan, A.A.; Halawa, M.; Saleh, F.M.; Saad, H.A.; Saied, E.M.; Fouda, A. The catalytic activity of biosynthesized magnesium oxide nanoparticles (MgO-NPs) for inhibiting the growth of pathogenic microbes, tanning effluent treatment, and chromium ion removal. Catalysts 2021, 11, 821.
- Altaf, M.; Zeyad, M.T.; Hashmi, M.A.; Manoharadas, S.; Hussain, S.A.; Abuhasil, M.S.A.; Almuzaini, M.A.M. Effective inhibition and eradication of pathogenic biofilms by titanium dioxide nanoparticles synthesized using Carum copticum extract. RSC Adv. 2021, 11, 19248–19257.
- Zubair, M.; Husain, F.M.; Qais, F.A.; Alam, P.; Ahmad, I.; Albalawi, T.; Ahmad, N.; Alam, M.; Baig, M.H.; Dong, J.-J. Bio-fabrication of titanium oxide nanoparticles from Ochradenus arabicus to obliterate biofilms of drug-resistant Staphylococcus aureus and Pseudomonas aeruginosa isolated from diabetic foot infections. Appl. Nanosci. 2021, 11, 375–387.
- Devlin, H.; Fulaz, S.; Hiebner, D.W.; O’Gara, J.P.; Casey, E. Enzyme-functionalized mesoporous silica nanoparticles to target Staphylococcus aureus and disperse biofilms. Int. J. Nanomed. 2021, 16, 1929–1942.
- Aguilera-Correa, J.J.; Gisbert-Garzarán, M.; Mediero, A.; Fernández-Aceñero, M.J.; de-Pablo-Velasco, D.; Lozano, D.; Esteban, J.; Vallet-Regí, M. Antibiotic delivery from bone-targeted mesoporous silica nanoparticles for the treatment of osteomyelitis caused by methicillin-resistant Staphylococcus aureus. Acta Biomater. 2022, 154, 608–625.
- Nie, B.e.; Huo, S.; Qu, X.; Guo, J.; Liu, X.; Hong, Q.; Wang, Y.; Yang, J.; Yue, B. Bone infection site targeting nanoparticle-antibiotics delivery vehicle to enhance treatment efficacy of orthopedic implant related infection. Bioact. Mater. 2022, 16, 134–148.
- Zulkarnain, M.Z.; Tong, W.Y.; Tan, W.-N.; Leong, C.R.; Md Yusof, F.A.; Wahidin, S.; Attifah, N.R.; Zubaidah, S. p-Coumaric acid quantum dots inhibit beta lactam resistant foodborne microorganisms. Mater. Today Proc. 2020, 31, 48–53.
- Li, P.; Liu, S.; Cao, W.; Zhang, G.; Yang, X.; Gong, X.; Xing, X. Low-toxicity carbon quantum dots derived from gentamicin sulfate to combat antibiotic resistance and eradicate mature biofilms. Chem. Commun. 2020, 56, 2316–2319.
- Wang, H.; Lu, F.; Ma, C.; Ma, Y.; Zhang, M.; Wang, B.; Zhang, Y.; Liu, Y.; Huang, H.; Kang, Z. Carbon dots with positive surface charge from tartaric acid and m-aminophenol for selective killing of Gram-positive bacteria. J. Mater. Chem. B 2021, 9, 125–130.
- Liang, J.; Li, W.; Chen, J.; Huang, X.; Liu, Y.; Zhang, X.; Shu, W.; Lei, B.; Zhang, H. Antibacterial activity and synergetic mechanism of carbon dots against Gram-positive and -negative bacteria. ACS Appl. Bio. Mater. 2021, 4, 6937–6945.
- Kung, J.-C.; Tseng, I.T.; Chien, C.-S.; Lin, S.-H.; Wang, C.-C.; Shih, C.-J. Microwave assisted synthesis of negative-charge carbon dots with potential antibacterial activity against multi-drug resistant bacteria. RSC Adv. 2020, 10, 41202–41208.
- Kumar, R. Chapter 8—Lipid-based nanoparticles for drug-delivery systems. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–284.
- Walduck, A.; Sangwan, P.; Vo, Q.A.; Ratcliffe, J.; White, J.; Muir, B.W.; Tran, N. Treatment of Staphylococcus aureus skin infection in vivo using rifampicin loaded lipid nanoparticles. RSC Adv. 2020, 10, 33608–33619.
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286.
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49.
- Mitchell, S.L.; Carlson, E.E. Tiny things with enormous impact: Nanotechnology in the fight against infectious disease. ACS Infect. Dis. 2018, 4, 1432–1435.
- Pinheiro, M.; Magalhães, J.; Reis, S. Antibiotic interactions using liposomes as model lipid membranes. Chem. Phys. Lipids 2019, 222, 36–46.
- Dong, D.; Thomas, N.; Thierry, B.; Vreugde, S.; Prestidge, C.A.; Wormald, P.-J. Distribution and inhibition of liposomes on Staphylococcus aureus and Pseudomonas aeruginosa biofilm. PLoS ONE 2015, 10, e0131806.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102.
- Williams, P.E.; Crauwels, H.M.; Basstanie, E.D. Formulation and pharmacology of long-acting rilpivirine. Curr. Opin. HIV AIDS 2015, 10, 233–238.
- Berti, I.R.; Dell’Arciprete, M.L.; Dittler, M.L.; Miñan, A.; de Mele, M.F.L.; Gonzalez, M. Delivery of fluorophores by calcium phosphate-coated nanoliposomes and interaction with Staphylococcus aureus biofilms. Colloids Surf. B Biointerfaces 2016, 142, 214–222.
- Cui, H.; Li, W.; Li, C.; Vittayapadung, S.; Lin, L. Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling 2016, 32, 215–225.
- Zomorodian, K.; Veisi, H.; Mousavi, S.M.; Ataabadi, M.S.; Yazdanpanah, S.; Bagheri, J.; Mehr, A.P.; Hemmati, S.; Veisi, H. Modified magnetic nanoparticles by PEG-400-immobilized Ag nanoparticles (Fe(3)O(4)@PEG-Ag) as a core/shell nanocomposite and evaluation of its antimicrobial activity. Int. J. Nanomed. 2018, 13, 3965–3973.
- Hu, H.; Zhong, D.; Li, W.; Lin, X.; He, J.; Sun, Y.; Wu, Y.; Shi, M.; Chen, X.; Xu, F.; et al. Microalgae-based bioactive hydrogel loaded with quorum sensing inhibitor promotes infected wound healing. Nano Today 2022, 42, 101368.
- Ferreira, M.; Pinto, S.; Aires-da-Silva, F.; Bettencourt, A.; Aguiar, S.; Gaspar, M. Liposomes as a nanoplatform to improve the delivery of antibiotics into Staphylococcus aureus biofilms. Pharmaceutics 2021, 13, 321.
- Hsu, C.-Y.; Sung, C.T.; Aljuffali, I.A.; Chen, C.-H.; Hu, K.-Y.; Fang, J.-Y. Intravenous anti-MRSA phosphatiosomes mediate enhanced affinity to pulmonary surfactants for effective treatment of infectious pneumonia. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 215–225.
- Silva, N.C.; Silva, S.; Sarmento, B.; Pintado, M. Chitosan nanoparticles for daptomycin delivery in ocular treatment of bacterial endophthalmitis. Drug Deliv. 2015, 22, 885–893.
- Vyas, S.; Sihorkar, V.; Jain, S. Mannosylated liposomes for bio-film targeting. Int. J. Pharm. 2007, 330, 6–13.
- Rani, N.N.I.M.; Chen, X.Y.; Al-Zubaidi, Z.M.; Azhari, H.; Khaitir, T.M.N.; Ng, P.Y.; Buang, F.; Tan, G.C.; Wong, Y.P.; Said, M.M.; et al. Surface-engineered liposomes for dual-drug delivery targeting strategy against methicillin-resistant Staphylococcus aureus (MRSA). Asian J. Pharm. Sci. 2022, 17, 102–119.
- Liu, X.; Li, Z.; Wang, X.; Chen, Y.; Wu, F.; Men, K.; Xu, T.; Luo, Y.; Yang, L. Novel antimicrobial peptide–modified azithromycin-loaded liposomes against methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2016, 11, 6781.
- Sahin, N.O. Niosomes as nanocarrier systems. In Nanomaterials and Nanosystems for Biomedical Applications; Mozafari, M.R., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 67–81.
- Mirzaie, A.; Peirovi, N.; Akbarzadeh, I.; Moghtaderi, M.; Heidari, F.; Yeganeh, F.E.; Noorbazargan, H.; Mirzazadeh, S.; Bakhtiari, R. Preparation and optimization of ciprofloxacin encapsulated niosomes: A new approach for enhanced antibacterial activity, biofilm inhibition and reduced antibiotic resistance in ciprofloxacin-resistant methicillin-resistance Staphylococcus aureus. Bioorg. Chem. 2020, 103, 104231.
- Kashef, M.T.; Saleh, N.M.; Assar, N.H.; Ramadan, M.A. The antimicrobial activity of ciprofloxacin-loaded niosomes against ciprofloxacin-resistant and biofilm-forming Staphylococcus aureus. Infect. Drug Resist. 2020, 13, 1619.
- Grimaldi, N.; Andrade, F.; Segovia, N.; Ferrer-Tasies, L.; Sala, S.; Veciana, J.; Ventosa, N. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 2016, 45, 6520–6545.
- Hassan, D.; Omolo, C.A.; Fasiku, V.O.; Elrashedy, A.A.; Mocktar, C.; Nkambule, B.; Soliman, M.E.; Govender, T. Formulation of pH-responsive quatsomes from quaternary bicephalic surfactants and cholesterol for enhanced delivery of vancomycin against methicillin resistant Staphylococcus aureus. Pharmaceutics 2020, 12, 1093.
- Dong, D.; Thomas, N.; Ramezanpour, M.; Psaltis, A.J.; Huang, S.; Zhao, Y.; Thierry, B.; Wormald, P.-J.; Prestidge, C.A.; Vreugde, S. Inhibition of Staphylococcus aureus and Pseudomonas aeruginosa biofilms by quatsomes in low concentrations. Exp. Biol. Med. 2020, 245, 34–41.
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162.
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269.
- Dalili, D.; Amini, M.; Faramarzi, M.A.; Fazeli, M.R.; Khoshayand, M.R.; Samadi, N. Isolation and structural characterization of Coryxin, a novel cyclic lipopeptide from Corynebacterium xerosis NS5 having emulsifying and anti-biofilm activity. Colloids Surf. B Biointerfaces 2015, 135, 425–432.
- Wang, Z.; Liu, X.; Peng, Y.; Su, M.; Zhu, S.; Pan, J.; Shen, B.; Duan, Y.; Huang, Y. Platensimycin-encapsulated liposomes or micelles as biosafe nanoantibiotics exhibited strong antibacterial activities against methicillin-resistant Staphylococcus aureus infection in mice. Mol. Pharm. 2020, 17, 2451–2462.
- Guo, H.; Wang, Z.; Du, Q.; Li, P.; Wang, Z.; Wang, A. Stimulated phase-shift acoustic nanodroplets enhance vancomycin efficacy against methicillin-resistant Staphylococcus aureus biofilms. Int. J. Nanomed. 2017, 12, 4679.
- Argenziano, M.; Banche, G.; Luganini, A.; Finesso, N.; Allizond, V.; Gulino, G.R.; Khadjavi, A.; Spagnolo, R.; Tullio, V.; Giribaldi, G. Vancomycin-loaded nanobubbles: A new platform for controlled antibiotic delivery against methicillin-resistant Staphylococcus aureus infections. Int. J. Pharm. 2017, 523, 176–188.
- Durham, P.G.; Sidders, A.E.; Beam, J.E.; Kedziora, K.M.; Dayton, P.A.; Conlon, B.P.; Papadopoulou, V.; Rowe, S.E. Harnessing ultrasound-stimulated phase change contrast agents to improve antibiotic efficacy against methicillin-resistant Staphylococcus aureus biofilms. Biofilm 2021, 3, 100049.
- Anjum, M.M.; Patel, K.K.; Dehari, D.; Pandey, N.; Tilak, R.; Agrawal, A.K.; Singh, S. Anacardic acid encapsulated solid lipid nanoparticles for Staphylococcus aureus biofilm therapy: Chitosan and DNase coating improves antimicrobial activity. Drug Deliv. Transl. Res. 2021, 11, 305–317.
- Fazly Bazzaz, B.; Khameneh, B.; Namazi, N.; Iranshahi, M.; Davoodi, D.; Golmohammadzadeh, S. Solid lipid nanoparticles carrying Eugenia caryophyllata essential oil: The novel nanoparticulate systems with broad-spectrum antimicrobial activity. Lett. Appl. Microbiol. 2018, 66, 506–513.
- Luan, L.; Chi, Z.; Liu, C. Chinese white wax solid lipid nanoparticles as a novel nanocarrier of curcumin for inhibiting the formation of Staphylococcus aureus biofilms. Nanomaterials 2019, 9, 763.
- Khalid, M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691.
- Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Oshi, M.A.; Naeem, M.; Ki, M.-H.; Lee, B.L.; Jung, Y.; Yoo, J.-W. Bacteria-targeted clindamycin loaded polymeric nanoparticles: Effect of surface charge on nanoparticle adhesion to MRSA, antibacterial activity, and wound healing. Pharmaceutics 2019, 11, 236.
- Thomas, N.; Thorn, C.; Richter, K.; Thierry, B.; Prestidge, C. Efficacy of poly-lactic-co-glycolic acid micro-and nanoparticles of ciprofloxacin against bacterial biofilms. J. Pharm. Sci. 2016, 105, 3115–3122.
- Manukumar, H.; Chandrasekhar, B.; Rakesh, K.; Ananda, A.; Nandhini, M.; Lalitha, P.; Sumathi, S.; Qin, H.-L.; Umesha, S. Novel AgNPs mediated biocidal mechanism against biofilm associated methicillin-resistant Staphylococcus aureus (Bap-MRSA) 090, cytotoxicity and its molecular docking studies. MedChemComm 2017, 8, 2181–2194.
- Zhang, X.; Manukumar, H.; Rakesh, K.; Karthik, C.; Prasad, H.N.; Swamy, S.N.; Mallu, P.; Mohammed, Y.H.E.; Qin, H.-L. Role of BP* AgNPs in Bap-dependent multicellular behavior of clinically important methicillin-resistant Staphylococcus aureus (MRSA) biofilm adherence: A key virulence study. Microb. Pathog. 2018, 123, 275–284.
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7, 1580.
- Siddhardha, B.; Pandey, U.; Kaviyarasu, K.; Pala, R.; Syed, A.; Bahkali, A.H.; Elgorban, A.M. Chrysin-Loaded Chitosan Nanoparticles Potentiates Antibiofilm Activity against Staphylococcus aureus. Pathogens 2020, 9, 115.
- Asli, A.; Brouillette, E.; Ster, C.; Ghinet, M.G.; Brzezinski, R.; Lacasse, P.; Jacques, M.; Malouin, F. Antibiofilm and antibacterial effects of specific chitosan molecules on Staphylococcus aureus isolates associated with bovine mastitis. PLoS ONE 2017, 12, e0176988.
- Breser, M.L.; Felipe, V.; Bohl, L.P.; Orellano, M.S.; Isaac, P.; Conesa, A.; Rivero, V.E.; Correa, S.G.; Bianco, I.D.; Porporatto, C. Chitosan and cloxacillin combination improve antibiotic efficacy against different lifestyle of coagulase-negative Staphylococcus isolates from chronic bovine mastitis. Sci. Rep. 2018, 8, 5081.
- Jamil, B.; Habib, H.; Abbasi, S.A.; Ihsan, A.; Nasir, H.; Imran, M. Development of cefotaxime impregnated chitosan as nano-antibiotics: De novo strategy to combat biofilm forming multi-drug resistant pathogens. Front. Microbiol. 2016, 7, 330.
- Silva, J.; Vanat, P.; Marques-da-Silva, D.; Rodrigues, J.R.; Lagoa, R. Metal alginates for polyphenol delivery systems: Studies on crosslinking ions and easy-to-use patches for release of protective flavonoids in skin. Bioact. Mater. 2020, 5, 447–457.
- Scolari, I.; Paez, P.; Musri, M.; Petiti, J.; Torres, A.; Granero, G. Rifampicin loaded in alginate/chitosan nanoparticles as a promising pulmonary carrier against Staphylococcus aureus. Drug Deliv. Transl. Res. 2020, 10, 1403–1417.
- Pavelková, M.; Vysloužil, J.; Kubová, K.; Pavloková, S.; Molinková, D.; Celer, V.; Pechová, A.; Mašek, J.; Vetchý, D. Assessment of antimicrobic, antivirotic and cytotoxic potential of alginate beads cross-linked by bivalent ions for vaginal administration. Pharmaceutics 2021, 13, 165.
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145.
- Malkoch, M.; García-Gallego, S. Introduction to dendrimers and other dendritic polymers. In Dendrimer Chemistry: Synthetic Approaches Towards Complex Architectures; The Royal Society of Chemistry: London, UK, 2020; pp. 1–20.
- Liu, X.; Wang, Z.; Feng, X.; Bai, E.; Xiong, Y.; Zhu, X.; Shen, B.; Duan, Y.; Huang, Y. Platensimycin-encapsulated poly(lactic-co-glycolic acid) and poly(amidoamine) dendrimers nanoparticles with enhanced anti-staphylococcal activity in vivo. Bioconjugate Chem. 2020, 31, 1425–1437.
- Gholami, M.; Mohammadi, R.; Arzanlou, M.; Dourbash, F.A.; Kouhsari, E.; Majidi, G.; Mohseni, S.M.; Nazari, S. In vitro antibacterial activity of poly (amidoamine)-G7 dendrimer. BMC Infect. Dis. 2017, 17, 395.
- Jiang, G.; Liu, S.; Yu, T.; Wu, R.; Ren, Y.; van der Mei, H.C.; Liu, J.; Busscher, H.J. PAMAM dendrimers with dual-conjugated vancomycin and Ag-nanoparticles do not induce bacterial resistance and kill vancomycin-resistant Staphylococci. Acta Biomater. 2021, 123, 230–243.
- Svenningsen, S.W.; Frederiksen, R.F.; Counil, C.; Ficker, M.; Leisner, J.J.; Christensen, J.B. Synthesis and antimicrobial properties of a ciprofloxacin and pamam-dendrimer conjugate. Molecules 2020, 25, 1389.
- Omolo, C.A.; Kalhapure, R.S.; Agrawal, N.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. A hybrid of mPEG-b-PCL and G1-PEA dendrimer for enhancing delivery of antibiotics. J. Control. Release 2018, 290, 112–128.
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N.; Overy, D.P.; Lanteigne, M.; McQuillan, K.; Kerr, R.G. Antimicrobial Organometallic Dendrimers with Tunable Activity against Multidrug-Resistant Bacteria. Biomacromolecules 2015, 16, 3694–3703.
- Thissa, N. Siriwardena, Michaela Stach, Runze He, Bee-Ha Gan, Sacha Javor, Marc Heitz, Lan Ma, Xiangju Cai, Peng Chen, Dengwen Wei, Hongtao Li, Jun Ma, Thilo Köhler, Christian van Delden, Tamis Darbre, and Jean-Louis Reymond. Lipidated Peptide Dendrimers Killing Multidrug-Resistant Bacteria. J. Am. Chem. Soc. 2018, 140, 423–432.
- Pinto, S.N.; Mil-Homens, D.; Pires, R.F.; Alves, M.M.; Serafim, G.; Martinho, N.; Melo, M.; Fialho, A.M.; Bonifácio, V.D. Core-shell polycationic polyurea pharmadendrimers: New-generation of sustainable broad-spectrum antibiotics and antifungals. Biomater. Science 2022, 10, 5197–5207.
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, E329–E357.
- Jaiswal, S.; Duffy, B.; Jaiswal, A.K.; Stobie, N.; McHale, P. Enhancement of the antibacterial properties of silver nanoparticles using β-cyclodextrin as a capping agent. Int. J. Antimicrob. Agents 2010, 36, 280–283.
- Rajewski, R.A.; Stella, V.J. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J. Pharm. Sci. 1996, 85, 1142–1169.
- Lin, L.; Mao, X.; Sun, Y.; Cui, H. Antibacterial mechanism of artemisinin/beta-cyclodextrins against methicillin-resistant Staphylococcus aureus (MRSA). Microb. Pathog. 2018, 118, 66–73.
- Pinho, E.; Soares, G.; Henriques, M. Evaluation of antibacterial activity of caffeic acid encapsulated by β-cyclodextrins. J. Microencapsul. 2015, 32, 804–810.
- Oliveira, F.d.S.; Freitas, T.S.d.; Cruz, R.P.d.; Costa, M.d.S.; Pereira, R.L.S.; Quintans-Júnior, L.J.; Andrade, T.d.A.; Menezes, P.d.P.; Sousa, B.M.H.d.; Nunes, P.S.; et al. Evaluation of the antibacterial and modulatory potential of α-bisabolol, β-cyclodextrin and α-bisabolol/β-cyclodextrin complex. Biomed. Pharmacother. 2017, 92, 1111–1118.
- Tometri, S.S.; Ahmady, M.; Ariaii, P.; Soltani, M.S. Extraction and encapsulation of Laurus nobilis leaf extract with nano-liposome and its effect on oxidative, microbial, bacterial and sensory properties of minced beef. J. Food Meas. Charact. 2020, 14, 3333–3344.
- Bhatia, E.; Sharma, S.; Jadhav, K.; Banerjee, R. Combinatorial liposomes of berberine and curcumin inhibit biofilm formation and intracellular methicillin resistant Staphylococcus aureus infections and associated inflammation. J. Mater. Chem. B 2021, 9, 864–875.
- Zafari, M.; Adibi, M.; Chiani, M.; Bolourchi, N.; Barzi, S.M.; Nosrati, M.S.S.; Bahari, Z.; Shirvani, P.; Noghabi, K.A.; Ebadi, M. Effects of cefazolin-containing niosome nanoparticles against methicillin-resistant Staphylococcus aureus biofilm formed on chronic wounds. Biomed. Mater. 2021, 16, 035001.
- Alanchari, M.; Mohammadi, M.; Yazdian, F.; Ahangari, H.; Ahmadi, N.; Emam-Djomeh, Z.; Homayouni-Rad, A.; Ehsani, A. Optimization and antimicrobial efficacy of curcumin loaded solid lipid nanoparticles against foodborne bacteria in hamburger patty. J. Food Sci. 2021, 86, 2242–2254.
- Da Costa, D.; Exbrayat-Héritier, C.; Rambaud, B.; Megy, S.; Terreux, R.; Verrier, B.; Primard, C. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J. Nanobiotechnol. 2021, 19, 12.
- Song, X.; Wang, L.; Liu, T.; Liu, Y.; Wu, X.; Liu, L. Mandarin (Citrus reticulata L.) essential oil incorporated into chitosan nanoparticles: Characterization, anti-biofilm properties and application in pork preservation. Int. J. Biol. Macromol. 2021, 185, 620–628.
- Asghar, M.A.; Yousuf, R.I.; Shoaib, M.H.; Asghar, M.A. Antibacterial, anticoagulant and cytotoxic evaluation of biocompatible nanocomposite of chitosan loaded green synthesized bioinspired silver nanoparticles. Int. J. Biol. Macromol. 2020, 160, 934–943.
