Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Andrea Piccioni.
The gut microbiota is a complex ecosystem consisting of bacteria, fungi, archaea, and viruses living in symbiosis with the human organism. This collection of bacteria, archaea, and eukaryotes that colonize the digestive system has developed this fascinating symbiotic relationship with its host over thousands of years, characterized by a complex mutually beneficial interaction.
- gut microbiota
- type 1 diabetes mellitus
- LADA
1. The Gut Microbiota and the Intestinal Barrier
The total number of these microorganisms is estimated to be between 1013 and 1014, a number close to that of all cells in the human body [2][1]. Moreover, the genetic makeup of all these microorganisms, called the microbiome, turns out to be even greater than that of humans [3][2].
Only in recent years, supported by some very important discoveries, and with the fundamental contribution of metagenomics and 16S ribosomal RNA gene sequencing, has the composition and numerous functions of the gut microbiota been better studied and understood [4][3].
The gut microbiota is physiologically composed of Firmicutes, Bacteroides, Proteobacteria, Actinobacteria, Euryarchaeota, and Verrucomicrobia [5][4] (Figure 1).
Figure 1. The main phyla constituting the gut microbiota.
The most represented bacterial phyla are Bacteriodetes and Firmicutes, which make up more than 90 percent of the gut microbiota [6][5].
The intestinal barrier constitutes a protective defense against pathogens, and toxic and dietary compounds [7][6]. It is formed by an external epithelial layer and an inner endothelial layer which form the gut epithelial and vascular barrier, respectively [8][7]. The microbiota reside in the gut lumen at an adequate distance from gut the mucosa [9][8].
In some contexts, this barrier function is lost in what is called Leaky Gut Syndrome, a condition that has been increasingly discussed lately, which some authors say contributes to the onset of major autoimmune diseases such as celiac disease, rheumatoid arthritis, multiple sclerosis, and type 1 diabetes mellitus [10][9].
The development of the gut microbiota is one of the most interesting and current fields of research. It is certainly affected by environmental influences, the best known of which is the diet [11][10].
As a confirmation of the symbiotic role between the gut microbiota and the host, early microbiota colonization right after birth deeply influences immunological, metabolic, and allergic diseases [12][11].
The gut microbiota physiologically plays a symbiotic role with its host, in which it is involved in numerous functions. For example, it plays an immune role in the defense against pathogens. It also plays a key role in nutrition, being involved in the extraction of short-chain fatty acids (SCFAs) and amino acids from foods [13][12].
Unhealthy diets and obesity are widely known as risk factors for the development of type 2 diabetes [14][13]. Alterations in the gut microbiota and their repercussions at the metabolic level play a key role in the development of type 2 diabetes mellitus [15,16][14][15]. Changes in microbiota composition and gut dysbiosis increase gut inflammation and directly induce endotoxemia, which is recognized as a conditioning promoter of infectious, chronic, and metabolic diseases and has also gained importance in cancer pathogenesis [17][16]. Recent studies have highlighted the link between type 2 diabetes mellitus and metabolic endotoxemia. In type 2 diabetes mellitus, metabolic endotoxins are associated with a worsening of glycemic levels [18][17].
Dysbiosis in the microbiota is associated, with varying degrees of evidence, with a large number of diseases, affecting the gastrointestinal system and others. Those affecting the digestive system include inflammatory bowel disease and irritable bowel syndrome; other disorders include metabolic diseases such as obesity and diabetes, allergic diseases, and neurodevelopmental diseases [19][18].
2. Diabetes Mellitus and Gut Microbiota
2.1. Early Evidence
Researchers have long hypothesized that patients with type 1 diabetes mellitus might have a different composition of the gut microbiota than that of healthy people [32][19]. This hypothesis is related to the finding of an increased incidence of type 1 diabetes mellitus diagnoses in developed countries, so it was thought that environmental factors might be involved in the pathogenesis [33][20]. The hygiene hypothesis, which correlates improved hygiene conditions with increased incidences of autoimmune diseases, has been known for some time. In experiments conducted on laboratory mice, an increased incidence of diabetes was found in those raised in a germ-free environment [34][21]. It is known that there is a profound link between gut microbiota and the immune system, the dysregulation of which underlies many important diseases. Dysbiosis is hypothesized to be the cause of dysregulation of the development of the inflammatory response underlying the onset of inflammatory bowel disease [35][22]. Dysregulation of the immune system is also implicated in the onset of other major autoimmune-type diseases, such as rheumatoid arthritis, psoriasis, multiple sclerosis, and type 1 diabetes mellitus [36][23]. This evidence is supported by another paper from 2018 [37][24] that started from the premise that the incidence of diabetes mellitus type 1 has been increasing in recent years, as have those of other immune-related diseases such as celiac disease and allergic diseases. To explain this phenomenon, the authors hypothesize that underlying it is a decrease in the presence of certain microorganisms, such as Bifidobacterium infantis, which plays a critically important role in breast milk metabolism, due to several reasons such as an increase in cesarean deliveries. This observation is reinforced by another important study conducted in Denmark [38][25], in which an increased incidence rate of type 1 diabetes mellitus in children who used broad-spectrum antibiotics in the first two years of life was found to be correlated with the mode of delivery. Additionally, it was found that infants delivered by cesarean section and treated with antibiotics had an increased risk of developing type 1 diabetes mellitus. These data reveal the key role played by the gut microbiota from the earliest moments of life. WResearchers have seen how its alteration could have such repercussions as to lead to the development of such an important disease as diabetes mellitus. Alterations, as already discussed, can of course come to develop after broad-spectrum antibiotic therapy, just as is the case with the onset of clostridium difficile infection or even secondary to the mode of delivery, again underscoring the key role played by the microbiota from the earliest moments of life.2.2. The Link between Onset of Diabetes Mellitus Type 1 and Gut Microbiota
Some important findings help uresearchers to better understand the link between type 1 diabetes mellitus and the gut microbiota. In autoantibody-positive patients, an increase in the proportion of bacteria belonging to the phylum Bacteroidetes was found, while in healthy subjects, that of Firmicutes was found to be increased [39][26]. This interesting researticlech summarizes the main evidence regarding the different compositions of the gut microbiota between patients with type 1 diabetes mellitus and healthy patients [40][27] (Figure 2).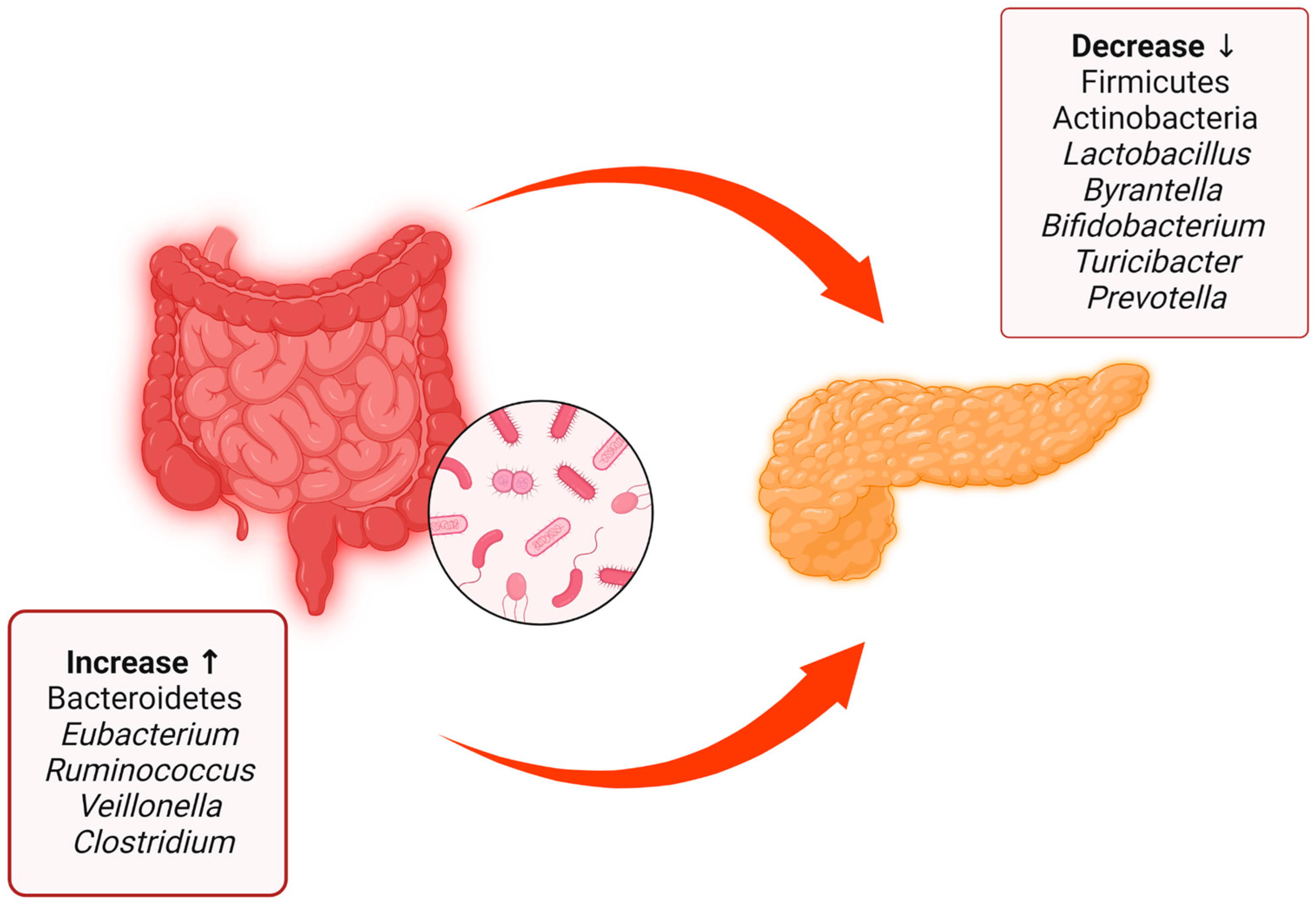
Figure 2. Summary of the main differences between the gut microbiota of patients with type 1 diabetes mellitus and healthy patients, with data obtained from both human patients and rats. There is a decrease in some bacterial species and phyla such as Firmicutes, Actinobacteria, Lactobacillus, Byrantella, Bifidobacterium, Turicibacter, and Prevotella accompanied by an increase in Bacteroidetes, Eubacterium, Ruminococcus, Veillonella, and Clostridium.
2.3. The Role of Short-Chain Fatty Acids and HLA in Diabetes Pathogenesis
There is a complex interplay linking diabetes pathogenesis, and the microbiota and their metabolic products. With regards to these products, short-chain fatty acids (SCFAs) play a pivotal role. Short-chain fatty acids, including butyrate, are products of fermentation of food residues. Butyrate appears to play a key role in metabolism, modulating satiety, blood sugar, and cholesterolemia [48][35]. The report that short-chain fatty acids play a protective role against diet-induced obesity and insulin resistance has been described in the literature [49][36]. In addition, oral administration of sodium butyrate in mice significantly increased plasma insulin [50][37]. According to some theories, diabetes mellitus type 1 may develop due to an alteration of the intestinal epithelial barrier, in the modulation of which short-chain fatty acids play a key role. In experiments in non-obese diabetic mouse models, there was an enhancement of beta cells in those that received butyrate and acetate [51,52][38][39]. In light of this evidence, the possible role of prebiotics and fecal microbiota transplantation in preventing diabetes complications has been suggested [53,54][40][41]. The microbiota may also, through the production of short-chain fatty acids, have an impact on diabetes complications such as atherosclerosis and peripheral artery disease, as SCFAs are believed to reduce inflammation and thus have a negative effect on the formation of atherosclerosis [55][42]. Some very other interesting piece of evidence comes from the TEDDY (the Environmental Determinants of Diabetes in the Young). The TEDDY study, among its many areas of research, was also interested in the relationship between the onset of juvenile diabetes and the gut microbiota. This study revealed the protective role of short-chain fatty acids against diabetes mellitus type 1 [56][43]. Type 1 diabetes mellitus falls among the autoimmune diseases, and similar to other autoimmune disorders, there is a close association with genetic factors. Many researchers have wondered whether there might be a link between HLA (human leukocyte antigen) genotypes and the development of the gut microbiota. A landmark study, conducted in Sweden over two years, involved infants whose fecal samples were collected at one year of age, the time when the autoimmunity that characterizes type 1 diabetes mellitus typically develops. Fecal composition was correlated with HLA genotype, and it was found that genotype may have an association with the gut microbiota of infants, resulting in the first study in which HLA genotypes were found to correlate with changes in the gut microbiota in human patients [57][44].2.4. The Use of Probiotics and Fecal Microbiota Transplantation as Treatment for Type 1 Diabetes Mellitus
Probiotics are defined as microorganisms that are beneficial to the body, while prebiotics are non-digestible foods that stimulate the growth of certain bacterial groups in the intestinal tract [58][45]. Attempts have been made in recent years to modulate the composition of the microbiota through the use of these new therapeutic weapons for the treatment of many diseases, among which type 1 diabetes mellitus could not be left out. Some important evidence on the use of probiotics comes from a recent study conducted in India, in which probiotic-treated children saw improved glycemic control indicated by a reduction in HbA1c and insulin treatment requirement [59][46]. This represents an important starting point, although there is still a long way to go in this regard, and many studies are still needed. With the use of fecal microbiota transplantation (FMT), as opposed to probiotics, one goes about manipulating the composition of the gut microbiota in a significantly more invasive way. A randomized controlled trial conducted in 2020 by de Groot et al. [41][28] demonstrated that fecal microbiota transplantation can prolong beta-cell function in newly diagnosed patients with type 1 diabetes mellitus. Some other important evidence comes to uresearchers from a recent study in which two patients with type 1 diabetes mellitus underwent fecal microbiota transplantation [60][47]. The results were surprising; one of the two no longer required hypoglycemic treatment during the months of follow-up in the study, while the other discontinued insulin treatment, and only took oral hypoglycemic drugs. These patients underwent multiple sessions of fecal microbiota transplantation, indicating a kind of “reinforcing effect.” On the strength of this evidence and the multiple insights gained over time, fecal microbiota transplantation is being studied as a therapeutic strategy for diabetes mellitus as well. Just to tie in with ourthe statements, weresearchers cite the very recent case of a 24-year-old young man with type 1 diabetes mellitus and malnutrition successfully treated with fecal microbiota transplantation [61][48]. All of this evidence gives hope that better glycemic control can be achieved in the future through these new therapeutic strategies.References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533.
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71.
- Lepage, P.; Leclerc, M.; Joossens, M.; Mondot, S.; Blottiere, H.; Raes, J.; Ehrlich, D.; Doré, J. A metagenomic insight into our gut’s microbiome. Gut 2012, 62, 146–158.
- Madhogaria, B.; Bhowmik, P.; Kundu, A. Correlation between human gut microbiome and diseases. Infect. Med. 2022, 1, 180–191.
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65.
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834.
- Di Tommaso, N.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R. The Gut–Vascular Barrier as a New Protagonist in Intestinal and Extraintestinal Diseases. Int. J. Mol. Sci. 2023, 24, 1470.
- Fox, E.; Lyte, M. Variation in spatial organization of the gut microbiota along the longitudinal and transverse axes of the intestines. Arch. Microbiol. 2022, 204, 1–17.
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708.
- Rajilić-Stojanović, M.; Milivojević, V. Nutrition and gut microbiota. Microb. Health Dis. 2020, 2, e193.
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812.
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191.
- Awa, W.L.; Fach, E.; Krakow, D.; Welp, R.; Kunder, J.; Voll, A.; Zeyfang, A.; Wagner, C.; Schütt, M.; Boehm, B.; et al. Type 2 diabetes from pediatric to geriatric age: Analysis of gender and obesity among 120 183 patients from the German/Austrian DPV database. Eur. J. Endocrinol. 2012, 167, 245–254.
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 834485.
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590.
- Manilla, V.; Di Tommaso, N.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R. Endotoxemia and Gastrointestinal Cancers: Insight into the Mechanisms Underlying a Dangerous Relationship. Microorganisms 2023, 11, 267.
- Vera, I.M.; Tapia, M.S.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2018, 45, 122–131.
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803.
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for Type 1 Diabetes. PLoS ONE 2011, 6, e25792.
- Patterson, C.C.; Dahlquist, G.; Soltész, G.; Green, A.; on behalf of the EURODIAB ACE Study Group. Is childhood-onset Type I diabetes a wealth-related disease? An ecological analysis of European incidence rates. Diabetologia 2001, 44, B9–B16.
- Bach, J.-F. The Effect of Infections on Susceptibility to Autoimmune and Allergic Diseases. N. Engl. J. Med. 2002, 347, 911–920.
- Singh, B.; Read, S.; Asseman, C.; Malmström, V.; Mottet, C.; Stephens, L.A.; Stepankova, R.; Tlaskalova, H.; Powrie, F. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 2001, 182, 190–200.
- Chow, J.; Mazmanian, S.K. Getting the Bugs out of the Immune System: Do Bacterial Microbiota “Fix” Intestinal T Cell Responses? Cell Host Microbe 2009, 5, 8–12.
- Insel, R.; Knip, M. Prospects for primary prevention of type 1 diabetes by restoring a disappearing microbe. Pediatr. Diabetes 2018, 19, 1400–1406.
- Clausen, T.D.; Bergholt, T.; Bouaziz, O.; Arpi, M.; Eriksson, F.; Rasmussen, S.; Keiding, N.; Løkkegaard, E.C. Broad-Spectrum Antibiotic Treatment and Subsequent Childhood Type 1 Diabetes: A Nationwide Danish Cohort Study. PLoS ONE 2016, 11, e0161654.
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyoty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011, 5, 82–91.
- Han, H.; Li, Y.; Fang, J.; Liu, G.; Yin, J.; Li, T.; Yin, Y. Gut Microbiota and Type 1 Diabetes. Int. J. Mol. Sci. 2018, 19, 995.
- De Groot, P.; Nikolic, T.; Pellegrini, S.; Sordi, V.; Imangaliyev, S.; Rampanelli, E.; Hanssen, N.; Attaye, I.; Bakker, G.; Duinkerken, G.; et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 2020, 70, 92–105.
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 2018, 67, 1445–1453.
- Förster, C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008, 130, 55–70.
- Gomes, J.M.G.; Costa, J.d.A.; Alfenas, R.d.C.G. Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism 2017, 68, 133–144.
- Utzschneider, K.M.; Kratz, M.; Damman, C.J.; Hullarg, M. Mechanisms Linking the Gut Microbiome and Glucose Metabolism. J. Clin. Endocrinol. Metab. 2016, 101, 1445–1454.
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772.
- De Kort, S.; Keszthelyi, D.; Masclee, A.A.M. Leaky gut and diabetes mellitus: What is the link? Obes. Rev. 2011, 12, 449–458.
- Looijer-van Langen, M.A.; Dieleman, L.A. Prebiotics in chronic intestinal inflammation. Inflamm. Bowel Dis. 2009, 15, 454–462.
- Remely, M.; Aumueller, E.; Merold, C.; Dworzak, S.; Hippe, B.; Zanner, J.; Pointner, A.; Brath, H.; Haslberger, A.G. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene 2014, 537, 85–92.
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Bu-tyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240.
- Hand, T.W.; Dos Santos, L.M.; Bouladoux, N.; Molloy, M.J.; Pagán, A.J.; Pepper, M.; Maynard, C.L.; Elson, C.O., 3rd; Belkaid, Y. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science 2012, 337, 1553–1556.
- Mariño, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017, 18, 552–562.
- Snelson, M.; de Pasquale, C.; Ekinci, E.I.; Coughlan, M.T. Gut microbiome, prebiotics, intestinal permeability and diabetes compli-cations. Best Pr. Res. Clin. Endocrinol. Metab. 2021, 35, 101507.
- Woldeamlak, B.; Yirdaw, K.; Biadgo, B. Role of Gut Microbiota in Type 2 Diabetes Mellitus and Its Complications: Novel Insights and Potential Intervention Strategies. Korean J. Gastroenterol. 2019, 74, 314–320.
- Muradi, A.; Jasirwan, C.O.M.; Simanjuntak, C.D.; Pratama, D.; Suhartono, R.; Darwis, P.; Kekalih, A. The Correlation of Short-Chain Fatty Acids with Peripheral Arterial Disease in Diabetes Mellitus Patients. Life 2022, 12, 1464.
- Vatanen, T.; Franzosa, E.A.; Schwager, R.; Tripathi, S.; Arthur, T.D.; Vehik, K.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.-X.; et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018, 562, 589–594.
- Russell, J.T.; Roesch, L.F.W.; Ördberg, M.; Ilonen, J.; Atkinson, M.A.; Schatz, D.A.; Triplett, E.W.; Ludvigsson, J. Genetic risk for autoimmunity is associated with distinct changes in the human gut microbiome. Nat. Commun. 2019, 10, 1–12.
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211.
- Kumar, S.; Kumar, R.; Rohilla, L.; Jacob, N.; Yadav, J.; Sachdeva, N. A high potency multi-strain probiotic improves glycemic control in children with new-onset type 1 diabetes mellitus: A randomized, double-blind, and placebo-controlled pilot study. Pediatr. Diabetes 2021, 22, 1014–1022.
- He, L.; Chen, R.; Zhang, B.; Zhang, S.; Khan, B.A.; Zhu, D.; Wu, Z.; Xiao, C.; Chen, B.; Chen, F.; et al. Fecal microbiota transplantation treatment of autoimmune-mediated type 1 diabetes mellitus. Front. Immunol. 2022, 13, 4219.
- Xie, Y.-C.; Jing, X.-B.; Chen, X.; Chen, L.-Z.; Zhang, S.-H.; Cai, X.-B. Fecal microbiota transplantation treatment for type 1 diabetes mellitus with malnutrition: A case report. Ther. Adv. Chronic Dis. 2022, 13, 20406223221117449.
More
