Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Gabija Didžiokaitė and Version 2 by Dean Liu.
Oxidative stress (OS) plays an important role in a variety of physiological and pathological processes of the female reproductive system.T In recent years the relationship between OS and endometriosis has been of particular interest, and a theory has been suggested that OS may be a cause of endometriosis development.
- oxidative stress
- reactive oxygen species
- infertility
- unexplained infertility
- endometriosis
1. OS Association with Infertility
There are more than a few identified mechanisms through which OS may affect female fertility as ROS affects multiple physiological and pathological activities in the ovaries as well as in the peritoneal environment. Figure 1. The major sources of ROS are cytokines, macrophages, and leukocytes present in the follicular fluid microenvironment [1]. A specific amount of ROS is necessary as ROS are considered critical inducers of ovulation [1]. However, one of the ways female fertility might be affected by OS is by ROS effect on female germ cells. OS appears to be the main mechanism by which the postovulatory oocyte loses its developmental competence after ovulation [2]. A complex series of events, all driven by an increase in oxidative stress, makes postovulatory oocytes enter apoptosis and lose their functionality [2]. One typical sign of postovulatory oocyte aging, for instance, is zona pellucida induration, which can be triggered by exposure to OS [2]. Ovoperoxidase, which is found in the cortical granules that are discharged from the surface of the oocyte during an exocytotic process, is in turn powered by OS and facilitates oocyte aging [2]. Moreover, when ROS are produced in excess they can also damage oocyte’s DNA which may result in improper fertilization [2]. Studies have indicated that psychological stress and aging might cause oxidative imbalance [3]. Various studies have reported that an age-induced increase in oxidative stress is also related to the age-dependant increase in aneuploidy as OS was found to be involved in the non-dysjunction of chromosomes that characterize oocytes that have aged in vivo [2][3][4][5].
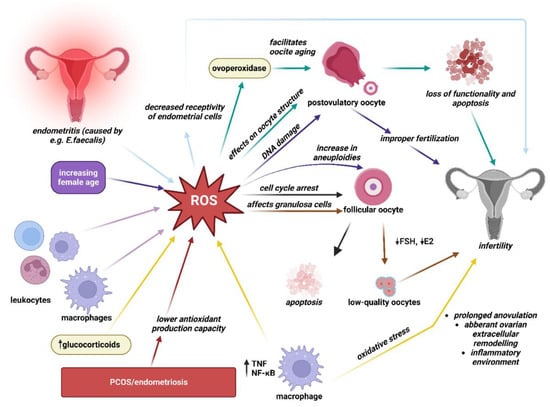
Figure 1. Identified mechanisms of oxidative stress-related infertility. The production of reactive oxygen species (ROS) during various processes (such as aging, uterine infections, etc.) and by various sources (such as leukocytes, macrophages, etc.) are illustrated via multi-colored arrows. Arrows of respective colors in turn represent the mechanisms by which ROSs may lead to infertility, e.g., E. faecalis endometritis may lead to an increase in ROS production, which in turn have an effect of decreasing receptivity of endometrial cells thus potentially leading to impaired fertility. (This figure was created with BioRender.com).
Furthermore, oxidative stress may have an effect on the granulosa cells’ ability to generate steroid hormones such as follicle-stimulating hormone (FSH) and estradiol (E2), which might affect the quality of oocytes [6]. The poor response to FSH and disturbed steroidogenic activity in older women may be connected to the increase in OS in the granulosa cells, which is associated with a decrease in the expression of the follicle-stimulating hormone receptor (FSHR) and a dysregulation of the FSHR signaling pathway. Women with endometriosis and PCOS have a lower antioxidant production capacity which may contribute to abnormal follicular development and infertility [6].
Some articles conclude that increased levels of stress hormones and decreased antioxidant activity augment the risk and prolong the duration of infertility [3]. The production of ROS beyond the physiological range (>80 ng/oocyte) is increased by stress-related pathological elevation of glucocorticoid levels. Excess ROS may lead to cell cycle arrest and apoptosis in follicular oocytes. They may affect not only ovarian but also uterine function, resulting in decreased fertilization and pregnancy rates [7].
Numerous studies have shown that PCOS patients also have increased OS. Patients with PCOS reported greater total oxidant status, higher serum prolidase activity, and higher OS indices, the proportion of oxidants to total antioxidant status. The mitochondrial malfunction in PCOS patients is explained by a reduction in mitochondrial O2 consumption and GSH levels as well as an increase in ROS generation. Increased levels of ROS produced by mononuclear cells during physiological hyperglycemia trigger the release of tumor necrosis factor (TNF) and increase the levels of a pro-inflammatory transcription factor, nuclear factor-kappa B (NF-κB). As a result, levels of TNF, a recognized modulator of insulin resistance, rise even more. The resulting OS induces prolonged anovulation, aberrant ovarian extracellular remodeling, cyst development, and an inflammatory environment that worsens insulin resistance, all of which contribute to infertility [1][8].
The association between higher levels of nitric oxide (NO) and nitric oxide synthase (NOS) with endometriosis and infertility has also been reported [9]. NO is a molecule of high physiological and pathological importance, whereas NOS is a family of enzymes catalyzing the production of NO [10]. NO is considered to be an essential molecule for normal reproductive biological processes such as sustaining pregnancy at physiological levels [9]. However, higher levels of NO have been reported to have harmful effects on sperm motility, toxicity to embryos, and inhibition of implantation [9]. It has been demonstrated that as the amount of NO in follicular fluid increases, the quality and rate of cleavage of the embryos are both reduced. Additionally, it has been noted that infertile women with tubal or peritoneal factor infertility had higher blood NO concentrations. Lower pregnancy rates are linked to follicular fluid NO concentrations that exceed the physiological limit, which can result in failed implantation. Studies conducted in vitro revealed that NO may even cause the embryo cells to undergo uncontrolled apoptosis and fragmentation [10].
Infections and ensuing oxidative stress are important factors in female reproductive health. For example, an opportunistic bacterium called Enterococcus faecalis is frequently discovered in the endometrium of patients with chronic endometritis. Superoxide-producing E. faecalis OG1RF infection of endometrial epithelial cells causes the production of inflammatory cytokines, encourages apoptosis, and suppresses the expression of receptivity indicators. Extracellular superoxide is a virulence factor of E. faecalis-induced endometritis that might result in decreased receptivity of endometrial epithelial cells and potentially lead to infertility [11].
Diminished ovarian reserve (DOR) seems to be associated with oxidative stress as well. DOR describes a decline in the number of oocytes in the ovary, which results in reduced female fertility and abnormalities of the reproductive endocrine system. OS can also cause ovarian endocrine dysfunction and follicular atresia, which is a major factor in the decline of fertility among patients with DOR [12].
DOR is a commonly encountered problem in reproductive medicine. While the levels of OS markers in follicular fluid are closely related to the growth, development, and maturation of the oocyte, it has been observed that changes in the DOR-associated metabolites in the follicular fluid may indicate the quality of oocytes. DOR-associated metabolites are a class of oxidative metabolites known as oxylipins, or lipid mediators, which are formed by the autooxidation of polyunsaturated fatty acids such as arachidonic acid, linoleic acid, alpha-linolenic acid, docosahexaenoic acid, and eicosapentaenoic acid as well as by the enzymes cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP). Nearly all physiological processes in the body involve the signal-transduction molecules oxylipins, which also play a crucial regulatory role in the organism’s daily activities such as immunological defense, oxidative stress, inflammatory response, and endocrine control [13][14].
Recent studies suggested that there were substantial changes in oxidative lipid metabolites between DOR patients and patients with normal ovarian reserve. The results of the study revealed 15 differentially expressed oxylipin metabolites, associated with ovarian reserve function. These fifteen oxylipin metabolites (±20-HDoHE, ±5-iso PGF2α-VI, 12S-HHTrE, 15-deoxy-Δ12,14-PGJ2, 1a,1b-dihomo PGE2, 1a,1b-dihomo PGF2α, 20-COOH-AA, 20-HETE, 8S,15S-DiHETE, PGA2, PGD2, PGE1, PGF1α, PGF2α, and PGJ2) were found to be of lower concentrations in the follicular fluid of DOR patients than of those in the normal ovarian reserve group. Oxylipin metabolism disorders and the function of ovarian reserve function were found to be closely related by metabolomic analysis of follicular fluid. While arachidonic acid is known to be involved in the regulation of oocyte development and maturation, it was observed that differentially oxidized lipid metabolites were mainly concentrated in the arachidonic acid (AA) metabolic pathway, therefore its complex changes might be closely related to impaired follicular development, leading to decreased fertility in DOR patients [14].
2. OS Association with Endometriosis
Endometriosis is considered to be one of the most common infertility-associated diseases. The first theories of endometriosis development were introduced almost 100 years ago. To this day, its etiology is still debatable and is usually based on one of the three main theories: retrograde menstruation and implantation theory by Sampson, coelomic metaplasia theory by Mayer, and the theory of induction [15]. However, for more than twenty years researchers have paid attention to the possible oxidative stress association with endometriosis development [15]. A number of oxidative stress biomarkers collected from different sites such as serum, peritoneal fluid, follicular fluid, ovarian cortex, eutopic, and ectopic endometrial tissues of women diagnosed with endometriosis were examined in different studies [15]. Based on the tendencies found, endometriosis was then characterized as the inflammatory process leading to the overproduction of inflammatory mediators due to oxidative stress [16]. When researching the exact role of oxidative stress in the pathophysiological mechanisms of endometriosis development, it was discovered that oxidative stress may be involved in multiple aspects of the disease and its progression. Figure 2.
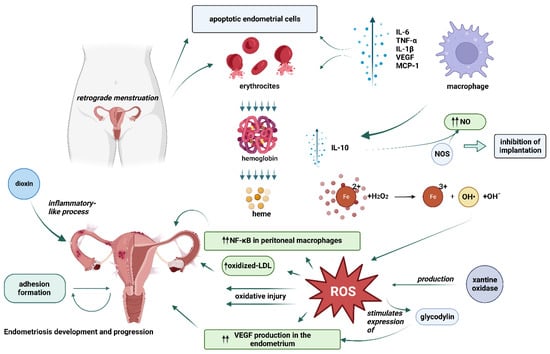
Figure 2. Potential mechanisms of oxidative stress related-endometriosis development. One of the many mechanisms is related to the activation of immune system caused by endometrial cells entering the peritoneal cavity during retrograde menstruation, which leads to excess reactive oxygen species (ROS) production by the activated macrophages, apoptotic endometrium cells, and erythrocytes. Iron, the by-product of hemolysis, then may act as a catalyst in the Fenton reaction further generating ROSs. Apart from direct oxidative injury to endometrial cells, ROSs can also increase the production of nuclear factor-kappa B (NF-κB) and vascular endothelial growth factor (VEGF) which play a role in the immune and inflammatory response in endometriotic cells and lead to an increase in levels of cytokines, chemokines, adhesion molecules, growth, and angiogenic factors all of which are related to the development and progression of endometriosis. (This figure was created with BioRender.com).
References
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. RBE 2018, 16, 80.
- Aitken, R.J. Impact of oxidative stress on male and female germ cells: Implications for fertility. Reproduction 2020, 159, R189–R201.
- Alam, F.; Khan, T.A.; Amjad, S.; Rehman, R. Association of oxidative stress with female infertility—A case control study. J. Pak. Med. Assoc. 2019, 69, 627–631.
- Perkins, A.T.; Greig, M.M.; Sontakke, A.A.; Peloquin, A.S.; McPeek, M.A.; Bickel, S.E. Increased levels of superoxide dismutase suppress meiotic segregation errors in aging oocytes. Chromosoma 2019, 128, 215–222.
- Mihalas, B.P.; Redgrove, K.A.; McLaughlin, E.A.; Nixon, B. Molecular Mechanisms Responsible for Increased Vulnerability of the Ageing Oocyte to Oxidative Damage. Oxidative Med. Cell. Longev. 2017, 2017, 4015874.
- Vila, J.; González-Fernández, R.; Rotoli, D.; Hernández, J.; Palumbo, A. Oxidative Stress in Granulosa-Lutein Cells From In Vitro Fertilization Patients. Reprod. Sci. 2016, 23, 1656–1661.
- Alam, F.; Khan, T.A.; Ali, R.; Tariq, F.; Rehman, R. SIRTI and cortisol in unexplained infertile females; a cross sectional study, in Karachi Pakistan. Taiwan. J. Obstet. Gynecol. 2020, 59, 189–194.
- Liu, Y.; Yu, Z.; Zhao, S.; Cheng, L.; Man, Y.; Gao, X.; Zhao, H. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J. Assist. Reprod. Genet. 2021, 38, 471–477.
- Ansariniya, H.; Yavari, A.; Javaheri, A.; Zare, F. Oxidative stress-related effects on various aspects of endometriosis. Am. J. Reprod. Immunol. 2022, 88, e13593.
- Dutta, S.; Sengupta, P. The Role of Nitric Oxide on Male and Female Reproduction. Malays J. Med. Sci. MJMS 2022, 29, 18–30.
- Zhang, Z.; Li, T.; Xu, L.; Wang, Q.; Li, H.; Wang, X. Extracellular superoxide produced by Enterococcus faecalis reduces endo-metrial receptivity via inflammatory injury. Am. J. Reprod. Immunol. 2021, 86, e13453.
- Razi, Y.; Eftekhar, M.; Fesahat, F.; Firouzabadi, R.D.; Razi, N.; Sabour, M.; Razi, M.H. Concentrations of homocysteine in follicular fluid and embryo quality and oocyte maturity in infertile women: A prospective cohort. J. Obstet. Gynaecol. 2020, 41, 588–593.
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540.
- Liang, C.; Zhang, X.; Qi, C.; Hu, H.; Zhang, Q.; Zhu, X.; Fu, Y. UHPLC-MS-MS analysis of oxylipins metabolomics components of follicular fluid in infertile individuals with diminished ovarian reserve. Reprod. Biol. Endocrinol. 2021, 19, 143.
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell Longev. 2017, 2017, 7265238.
- Amini, L.; Chekini, R.; Nateghi, M.R.; Haghani, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res. Manag. 2021, 2021, 5529741.
More
