Neurodegenerative diseases (NDs) are a diverse group of pathologies characterized by a gradual loss in neuron number and function. These pathologies are primarily caused by the accumulation of misfolded proteins, as seen in Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease, and are associated with a decline in cognitive abilities and movement disorders. There has been an increase in the study of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway and the natural products that positively regulate it to reduce oxidative damage to the nervous system, both in in vitro models with neurons and microglia subjected to stress factors and in vivo models using mainly murine models. Quercetin, curcumin, anthocyanins, tea polyphenols, and other less studied phenolic compounds such as kaempferol, hesperetin, and icariin can also modulate Nrf2 by regulating several Nrf2 upstream activators.
- Nrf2
- oxidative stress
- neurodegeneration
- phenolic compounds
1. Introduction
2. Nuclear Factor Erythroid 2-Related Factor 2 Signaling Pathway against OS
-
p62 phosphorylated at Ser-351: p62, also known as sequestosome 1, is a multifunctional protein involved in various cellular processes, including autophagy and OS response. Upon phosphorylation, p62 acquires high affinity for Keap-1, preventing Nrf2 ubiquitination and degradation [23].
-
Glycogen synthase kinase-3 (GSK-3): GSK-3 is a Ser/Thr kinase that negatively regulates Nrf2 by phosphorylating Ser residues of the Neh6 domain. The phosphorylated residues are recognized by the E3 adaptor ligase β-TrCP (β-transducin repeat-containing protein), which recruits the Cul3/Rbx complex, ubiquitinates the Nrf2, and leads to its degradation. The PI3K (phosphatidylinositol-3 kinase)/Akt (protein kinase B) pathway can inhibit GSK-3 and prevents Nrf2 phosphorylation. Likewise, PI3K/Akt can be activated by ion channels, growth factors, and G coupled-protein receptor ligands [24].
-
RXRα (retinoid X receptor α): this transcription factor associates with the Neh7 domain of Nrf2 to block the expression of genes related to decreased OS [25].
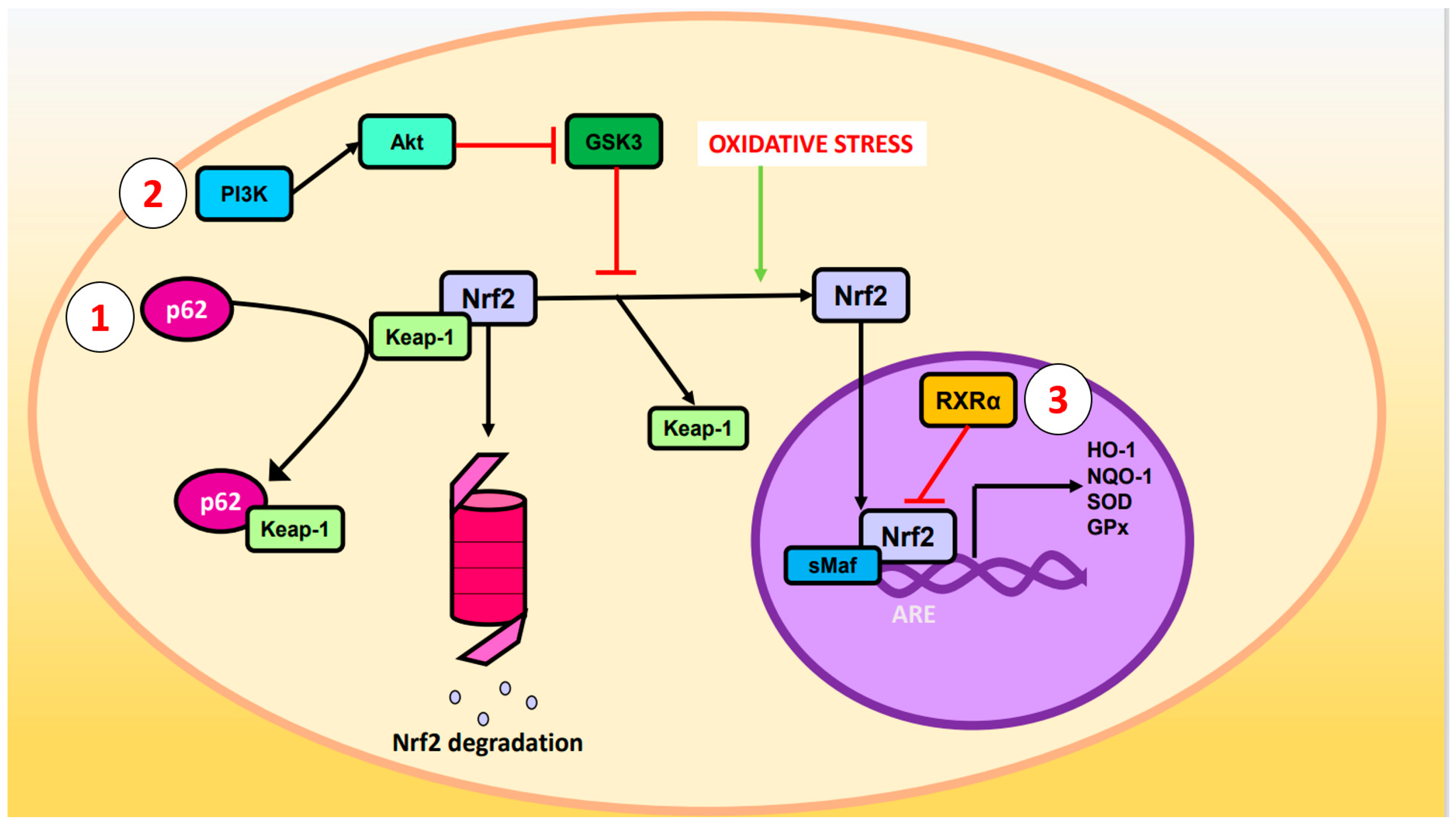
3. Phenolic Compounds
| Treatment | Experimental Model | Effects on Nrf2 Pathway | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Metanolic extract of Dendropanax morbifera leaves, quercetin, or isoquercetin | Glutamate-induced oxidative stress (OS) in HT22 cells. | Three treatments ↑ Nrf2 and HO-1 protein levels. | [27 | ||||
| Quercetin | Aβ42 supplied intracranially in SD rats. | ↑ Nrf2, HO-1, SOD, CAT, and GSH protein levels. | ] | ||||
| [ | 53 | ] | Quercetin | 7-ketocholecterol-induced oxiapoptophagy in N2a cells. | ↑ Nrf2 and SOD-1 mRNA levels. ↑ Nrf2, SOD-1, SOD-2, CAT, and GPx protein levels. ↑ SOD and GPx activities. |
[28] | |
| Streptozotocin supplied intracerebroventricularly in Wistar rats. | ↑ HO-1 protein levels (maybe mediated by α7nAChR/Nrf2 pathway). | Corticosterone-induced cytotoxicity in primary cortical neurons. | Protective effect of quercetin is not mediated by Nrf2. | [29] | |||
| [ | 54 | ] | |||||
| Energy-drink-induced neurotoxicity in Wistar rats. | ↑ Nrf2 and HO-1 mRNA and protein levels. | [55] | ΔK280 TauRD-DsRed SH-SY5Y cells. | ||||
| Traumatic brain injury in SD rats. | ↑ Nrf2 protein levels (maybe mediated by TRKB/Akt pathway). | ↑ Nrf2 nuclear and cytoplasmatic protein levels. ↑ HO-1 protein levels. ↑ SOD, CAT, and GPx activities.[30] |
|||||
| [ | 56 | ] | High glucose concentration in SH-SY5Y cells. | ↑ Nrf2 and p-NRF2 protein levels (maybe mediated by PKC activation and/or GSK-3β inhibition). | |||
| Chronic unpredictable mild stress in Kunming mice. | ↑ Nrf2 nuclear levels. |
↑ Nrf2, HO-1, p-PI3K, and p-Akt protein levels.[31] | |||||
| ↑ SOD and glutathione-S-transferase (GST) activities. | [57] | Aβ25-35-induced cytotoxicity in PC12 cells. | ↑ HO-1 mRNA and protein levels. ↓ Nrf2 and Sirtuin-1 (SIRT1) mRNA and protein levels. ↑ SOD, CAT, and GPx activities. |
[32] | |||
| Acrylamide-induced cytotoxicity in C6 cells. | |||||||
| t-MCAO in Wistar rats. | ↑ Nrf2, HO-1, and SIRT1 protein levels. | [58] | ↑ Nrf2 nuclear levels. | [33] | |||
| Curcumin | Traumatic brain injury in ICR mice. | ↑ HO-1 and NQO-1 protein levels. ↑ SOD and GPx activities. |
[59] | Curcumin | Immortalized mouse cortical neuronal cells. | ↑ Nrf2, HO-1, NQO-1, and GST mRNA and protein levels (maybe mediated by PKCδ/p62/Nrf2 pathway) ↑ Nrf2 nuclear levels. ↑ ARE activity. |
[34] |
| Traumatic brain injury in Nrf2-knockout mice and WT. | ↑ Nrf2, HO-1, and NQO-1 mRNA and protein levels in WT mice. ↓ HO-1 and NQO-1 mRNA and protein levels in Nrf2-knockout mice |
[60] | Ethanol-induced OS in HT22 cells. | ↑ Nrf2 and HO-1 protein levels. | [35] | ||
| ↑ Nrf2 nuclear levels. | |||||||
| Chronic unpredictable mild stress in SD rats. | ↑ Nrf2, HO-1, and NQO-1 mRNA levels. ↑ Nrf2 nuclear levels. |
[61] | Methylmercury-induced cytotoxicity in primary rat astrocytes. | ↑ Nrf2, HO-1, NQO-1 and GSH protein levels (independently of PKCδ). ↑ Nrf2 nuclear levels. ↑ CAT activity. |
[36] | ||
| Quinolinic-acid-induced neurotoxicity in Wistar rats. | ↑ Nrf2 protein levels (maybe mediated by BDNF/TRKB/ERK pathway). ↑ CAT, GSH, SOD, and GPx activities. |
[62] | Heme-induced OS in HAPI cells and primary rat cortical microglia. | ↑ Nrf2, HO-1, NQO-1, and GPx4 mRNA and protein levels. | |||
| Arsenic-trioxide-induced neurotoxicity in Sanshui white ducks. | ↑ Nrf2, SOD-1, HO-1, CAT, GPx1, and thioredoxin mRNA and protein levels. ↓Keap1 mRNA and protein levels. | [37] | |||||
| [ | 63 | ] | Pam3CSK4-stimulated BV2 cells | ↑ Nrf2 and HO-1 protein levels. ↑ Nrf2 nuclear levels. |
|||
| Olive dry extract enriched in hydroxytyrosol 20% | Caenorhabditis elegans wild-type and C. elegans mutants. | [38] | |||||
| ↑ SKIN-1 (same function that Nrf2). | [ | 64 | ] | Hydroxytyrosol | 6-OHDA-induced cytotoxicity in SH-SY5Y cells. | No changes in Nrf2 protein levels. No changes in HO-1 mRNA and protein levels. |
[39] |
| Anthocyanins purified from methanolic extract from Korean black bean | Double-mutant APP/PS1 mice as AD model. | ↑ Nrf2 nuclear levels (maybe mediated by PI3K/Akt/GSK-3β pathway). ↑ HO-1 and GCLM protein levels. |
[41] | Mixture of oleuropein, p-coumaric acid, and tyrosol. | H2O2-induced oxidative damage in SK-N-SH cells. | ↓ Nrf2 protein levels. | |
| Proanthocyanidins | Cypermethrin-induced OS in mouse cortical neurons. | ↓ Nrf2, HO-1, and NQO-1 mRNA and protein levels. ↑ Keap-1 protein levels. | [40] | ||||
| [ | 43 | ] | Anthocyanins purified from methanolic extract from Korean black bean | AβO-induced cytotoxicity in HT22 cells. | ↑ Nrf2, p-PI3K, p-Akt, p-GSK-3β, HO-1, and GCLM protein levels (reversed by Nrf2 and p-PI3K inhibitors). | ||
| Icariin | 6-OHDA-induced neuroinflammation in WT and Nrf2 knockout mice. | ↑ Nrf2, HO-1, and NQO-1 mRNA and protein levels in WT mice. ↑ Nrf2 nuclear levels in WT mice. Nrf2 knockout mice did not have these effects. | [41] | ||||
| [ | 47 | ] | Cyanidin-3-glucoside | Glutamate-induced cytotoxicity in HT22 cells. | ↑ Nrf2 and ERK protein levels. ↑ SOD-1, SOD-2, CAT, and GPx mRNA levels. |
[42] | |
| Hesperetin | Aβ1-42-induced neurotoxicity in brain mice. | ↑ Nrf2 and HO-1 protein level. | [65] | Proanthocyanidins | Cypermethrin-induced OS in mouse cortical neurons. | ||
| Ethanolic extract of Abelmoschus esculentus | ↓ Nrf2, HO-1, and NQO-1 mRNA and protein levels. | ↑ Keap-1 protein levels. |
flowers | TCIRI-induced OS in Kunming mice. | ↑ Nrf2, HO-1, and NQO-1 protein levels.[43] | ||
| [ | 66 | ] | Kaempferol | OGD/R-induced ferroptosis in primary mouse cortical neurons. | ↑ Nrf2, SLC7A11, and GPx4 protein levels. ↑ GSH and SOD activities. |
[ | |
| EGCG | CCH-induced cognitive impairments in SD rats. | 44 | ] | ||||
| ↑ Nrf2, HO-1, PI3K, p-Akt, and SOD protein levels. | ↑ HO-1 activity. | Tiliroside | BV2 and HT22 cells. | ↑ Nrf2, HO-1, NQO-1, and SIRT1 protein levels in BV2 cells. ↑ Nrf2, HO-1, and NQO-1 protein levels in HT22 cells. |
[45] | ||
| Engeletin | Aβ1-42-induced OS and neuroinflammation in BV2 cells. | ↑ Nrf2 protein levels. ↑ Nrf2 nuclear levels. ↓ Keap1 protein levels. ↑ SOD and GPx activities. |
[46] | ||||
| [ | 67 | ] | Icariin | 6-OHDA-induced neuroinflammation in mouse neuron–microglia co-culture. | Inhibition of Nrf2 reversed protective effect of icariin in microglia cells, but not in neurons. | [47] | |
| Isoliquiritigenin | AβO-induced neuroinflammation in BV2 cells. | ↑ Nrf2, HO-1, and NQO-1 mRNA and protein levels. | [48] | ||||
| Pinocembrin-7-methyleter | 6-OHDA-induced neurotoxicity in SH-SY5Y cells. | ↑ Nrf2 nuclear levels (maybe mediated by ERK and Akt). ↑ SOD and GPx activities. ↑HO-1 and NQO-1 mRNA and protein levels. |
[49] | ||||
| Garlic acid (GA), epigallocatechin (EGC), epicatechin-3-gallate (EGG), epigallocatechin-3-gallate (EGCG), theaflavin (TF), and tannic acid (TA) | HEK293T and SH-SY5Y cells. | GA, EGG, and TA ↑ ARE activity in HEK293T. GA, EGG, and TA ↑ HO-1 protein levels in SH-SY5Y cells. EGC, EGCG, and TF did not have these effects. |
[50] | ||||
| TF from ethanolic extract of black tea | OGD/R-induced OS in PC12 cells. | ↑ HO-1 protein levels. ↑ Nrf2 nuclear levels. ↑ SOD activity. |
[51] | ||||
| EGCG | CoCl2-induced hypoxia in BV2 cells. | ↑ HO-1 protein levels. ↑ Nrf2 nuclear levels. |
[52] |
| Treatment | Experimental Model | Effects on Nrf2 Pathway | Ref. |
|---|
3.2. Curcumin
3.3. Other Phenolic Compounds
3.3.1. Phenolic Compounds of Olive Tree
3.3.2. Anthocyanins and Proanthocyanidins
3.3.3. Flavonoids
3.3.4. Tea Polyphenols
References
- Alzheimer’s Disease International. World Alzheimer Report 2019. The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International: London, UK, 2019; pp. 1–86.
- Sumien, N.; Cunningham, J.T.; Davis, D.L.; Engelland, R.; Fadeyibi, O.; Farmer, G.E.; Mabry, S.; Mensah-Kane, P.; Trinh, O.T.P.; Vann, P.H.; et al. Neurodegenerative Disease: Roles for Sex, Hormones, and Oxidative Stress. Endocrinology 2021, 162, bqab185.
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581.
- Jurcau, A. Insights into the pathogenesis of neurodegenerative diseases: Focus on mitochondrial dysfunction and oxidative stress. Int. J. Mol. Sci. 2021, 22, 11847.
- Jacob, R.A. The integrated antioxidant system. Nutr. Res. 1995, 15, 755–766.
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules 2021, 11, 589.
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726.
- Martel, J.; Ojcius, D.M.; Ko, Y.F.; Ke, P.Y.; Wu, C.Y.; Peng, H.H.; Young, J.D. Hormetic effects of phytochemicals on health and longevity. Trends Endocrinol. Metab. 2019, 30, 335–346.
- Chen, W.; Jia, Z.; Pan, M.H.; Anandh Babu, P.V. Natural products for the prevention of oxidative stress-related diseases: Mechanisms and strategies. Oxid. Med. Cell Longev. 2016, 2016, 4628502.
- Velmurugan, B.K.; Rathinasamy, B.; Lohanathan, B.P.; Thiyagarajan, V.; Weng, C.F. Neuroprotective role of phytochemicals. Molecules 2018, 23, 2485.
- Paunkov, A.; Chartoumpekis, D.V.; Ziros, P.G.; Sykiotis, G.P. A bibliometric review of the Keap1/Nrf2 pathway and its related antioxidant compounds. Antioxidants 2019, 8, 353.
- Motohashi, H.; Yamamoto, M. Nrf2–Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557.
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930.
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745.
- Soares, M.P.; Ribeiro, A.M. Nrf2 as a master regulator of tissue damage control and disease tolerance to infection. Biochem. Soc. Trans. 2015, 43, 663–668.
- Taguchi, K.; Yamamoto, M. The KEAP1-NRF2 System in Cancer. Front. Oncol. 2017, 7, 85.
- Boas, S.M.; Joyce, K.L.; Cowell, R.M. The NRF2-Dependent Transcriptional Regulation of Antioxidant Defense Pathways: Relevance for Cell Type-Specific Vulnerability to Neurodegeneration and Therapeutic Intervention. Antioxidants 2021, 11, 8.
- Tong, K.I.; Katoh, Y.; Kusunoki, H.; Itoh, K.; Tanaka, T.; Yamamoto, M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition model. Mol. Cell Biol. 2006, 26, 2887–2900.
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radical Biol. Med. 2009, 47, 1304–1309.
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20.
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789.
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426.
- Ichimura, Y.; Waguri, S.; Sou, Y.S.; Kageyama, S.; Hasegawa, J.; Ishimura, R.; Saito, T.; Yang, Y.; Kouno, T.; Fukutomi, T.; et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell 2013, 51, 618–631.
- Cuadrado, A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radical Biol. Med. 2015, 88, 147–157.
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013, 73, 3097–3108.
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233.
- Park, H.J.; Kim, H.N.; Kim, C.Y.; Seo, M.D.; Baek, S.H. Synergistic Protection by Isoquercitrin and Quercetin against Glutamate-Induced Oxidative Cell Death in HT22 Cells via Activating Nrf2 and HO-1 Signaling Pathway: Neuroprotective Principles and Mechanisms of Dendropanax morbifera Leaves. Antioxidants 2021, 10, 554.
- Yammine, A.; Zarrouk, A.; Nury, T.; Vejux, A.; Latruffe, N.; Vervandier-Fasseur, D.; Samadi, M.; Mackrill, J.J.; Greige-Gerges, H.; Auezova, L.; et al. Prevention by Dietary Polyphenols (Resveratrol, Quercetin, Apigenin) Against 7-Ketocholesterol-Induced Oxiapoptophagy in Neuronal N2a Cells: Potential Interest for the Treatment of Neurodegenerative and Age-Related Diseases. Cells 2020, 9, 2346.
- Donoso, F.; Ramírez, V.T.; Golubeva, A.V.; Moloney, G.M.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Naturally Derived Polyphenols Protect Against Corticosterone-Induced Changes in Primary Cortical Neurons. Int. J. Neuropsychopharmacol. 2019, 22, 765–777.
- Chiang, N.N.; Lin, T.H.; Teng, Y.S.; Sun, Y.C.; Chang, K.H.; Lin, C.Y.; Hsieh-Li, H.M.; Su, M.T.; Chen, C.M.; Lee-Chen, G.J. Flavones 7,8-DHF, Quercetin, and Apigenin Against Tau Toxicity via Activation of TRKB Signaling in ΔK280 TauRD-DsRed SH-SY5Y Cells. Front Aging Neurosci. 2021, 13, 758895.
- Liu, Y.W.; Liu, X.L.; Kong, L.; Zhang, M.Y.; Chen, Y.J.; Zhu, X.; Hao, Y.C. Neuroprotection of quercetin on central neurons against chronic high glucose through enhancement of Nrf2/ARE/glyoxalase-1 pathway mediated by phosphorylation regulation. Biomed. Pharmacother. 2019, 109, 2145–2154.
- Yu, X.; Li, Y.; Mu, X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ25-35 and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. Biomed. Res. Int. 2020, 2020, 8210578.
- Kacar, S.; Sahinturk, V.; Tomsuk, O.; Kutlu, H.M. The effects of thymoquinone and quercetin on the toxicity of acrylamide in rat glioma cells. J. Biochem. Mol. Toxicol. 2022, 36, e22992.
- Park, J.Y.; Sohn, H.Y.; Koh, Y.H.; Jo, C. Curcumin activates Nrf2 through PKCδ-mediated p62 phosphorylation at Ser351. Sci. Rep. 2021, 11, 8430.
- Ikram, M.; Saeed, K.; Khan, A.; Muhammad, T.; Khan, M.S.; Jo, M.G.; Rehman, S.U.; Kim, M.O. Natural Dietary Supplementation of Curcumin Protects Mice Brains against Ethanol-Induced Oxidative Stress-Mediated Neurodegeneration and Memory Impairment via Nrf2/TLR4/RAGE Signaling. Nutrients 2019, 11, 1082.
- Yang, B.; Yin, C.; Zhou, Y.; Wang, Q.; Jiang, Y.; Bai, Y.; Qian, H.; Xing, G.; Wang, S.; Li, F.; et al. Curcumin protects against methylmercury-induced cytotoxicity in primary rat astrocytes by activating the Nrf2/ARE pathway independently of PKCδ. Toxicology 2019, 425, 152248.
- Duan, C.; Wang, H.; Jiao, D.; Geng, Y.; Wu, Q.; Yan, H.; Li, C. Curcumin Restrains Oxidative Stress of After Intracerebral Hemorrhage in Rat by Activating the Nrf2/HO-1 Pathway. Front. Pharmacol. 2022, 13, 889226.
- Jin, M.; Park, S.Y.; Shen, Q.; Lai, Y.; Ou, X.; Mao, Z.; Lin, D.; Yu, Y.; Zhang, W. Anti-neuroinflammatory effect of curcumin on Pam3CSK4-stimulated microglial cells. Int. J. Mol. Med. 2018, 41, 521–530.
- Funakohi-Tago, M.; Sakata, T.; Fujiwara, S.; Sakakura, A.; Sugai, T.; Tago, K.; Tamura, H. Hydroxytyrosol butyrate inhibits 6-OHDA-induced apoptosis through activation of the Nrf2/HO-1 axis in SH-SY5Y cells. Eur. J. Pharmacol. 2018, 834, 246–256.
- Lambert de Malezieu, M.; Courtel, P.; Sleno, L.; Abasq, M.L.; Ramassamy, C. Synergistic properties of bioavailable phenolic compounds from olive oil: Electron transfer and neuroprotective properties. Nutr. Neurosci. 2021, 24, 660–673.
- Ali, T.; Kim, T.; Rehman, S.U. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093.
- Sukprasansap, M.; Chanvorachote, P.; Tencomnao, T. Cyanidin-3-glucoside activates Nrf2-antioxidant response element and protects against glutamate-induced oxidative and endoplasmic reticulum stress in HT22 hippocampal neuronal cells. BMC Complement Med. Ther. 2020, 20, 46.
- Zhou, L.; Chang, J.; Zhao, W.; Gao, Y. Proanthocyanidins regulate the Nrf2/ARE signaling pathway and protect neurons from cypermethrin-induced oxidative stress and apoptosis. Pestic. Biochem. Physiol. 2021, 177, 104898.
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923.
- Velagapudi, R.; El-Bakoush, A.; Olajide, O.A. Activation of Nrf2 Pathway Contributes to Neuroprotection by the Dietary Flavonoid Tiliroside. Mol. Neurobiol. 2018, 55, 8103–8123.
- Huang, Z.; Ji, H.; Shi, J.; Zhu, X.; Zhi, Z. Engeletin Attenuates Aβ1-42-Induced Oxidative Stress and Neuroinflammation by Keap1/Nrf2 Pathway. Inflammation 2020, 43, 1759–1771.
- Zhang, B.; Wang, G.; He, J.; Yang, Q.; Li, D.; Li, J.; Zhang, F. Icariin attenuates neuroinflammation and exerts dopamine neuroprotection via an Nrf2-dependent manner. J. Neuroinflammation 2019, 16, 92.
- Fu, Y.; Jia, J. Isoliquiritigenin Confers Neuroprotection and Alleviates Amyloid-β42-Induced Neuroinflammation in Microglia by Regulating the Nrf2/NF-κB Signaling. Front. Neurosci. 2021, 15, 638772.
- Zou, Z.C.; Fu, J.J.; Dang, Y.Y.; Zhang, Q.; Wang, X.F.; Chen, H.B.; Jia, X.J.; Lee, S.M.; Li, C.W. Pinocembrin-7-Methylether Protects SH-SY5Y Cells Against 6-Hydroxydopamine-Induced Neurotoxicity via Modulating Nrf2 Induction Through AKT and ERK Pathways. Neurotox. Res. 2021, 39, 1323–1337.
- Zhou, Z.D.; Xie, S.P.; Saw, W.T.; Ho, P.G.H.; Wang, H.; Lei, Z.; Yi, Z.; Tan, E.K. The Therapeutic Implications of Tea Polyphenols Against Dopamine (DA) Neuron Degeneration in Parkinson’s Disease (PD). Cells 2019, 8, 911.
- Li, Y.; Shi, J.; Sun, X.; Li, Y.; Duan, Y.; Yao, H. Theaflavic acid from black tea protects PC12 cells against ROS-mediated mitochondrial apoptosis induced by OGD/R via activating Nrf2/ARE signaling pathway. J. Nat. Med. 2020, 74, 238–246.
- Kim, S.R.; Seong, K.J.; Kim, W.J.; Jung, J.Y. Epigallocatechin Gallate Protects against Hypoxia-Induced Inflammation in Microglia via NF-κB Suppression and Nrf-2/HO-1 Activation. Int. J. Mol. Sci. 2022, 23, 4004.
- Li, Y.; Tian, Q.; Li, Z.; Dang, M.; Lin, Y.; Hou, X. Activation of Nrf2 signaling by sitagliptin and quercetin combination against β-amyloid induced Alzheimer’s disease in rats. Drug Dev. Res. 2019, 80, 837–845.
- Singh, N.K.; Garabadu, D. Quercetin Exhibits α7nAChR/Nrf2/HO-1-Mediated Neuroprotection Against STZ-Induced Mitochondrial Toxicity and Cognitive Impairments in Experimental Rodents. Neurotox. Res. 2021, 39, 1859–1879.
- Sayed, W.M. Quercetin Alleviates Red Bull Energy Drink-Induced Cerebral Cortex Neurotoxicity via Modulation of Nrf2 and HO-1. Oxid. Med. Cell Longev. 2021, 2021, 9482529.
- Song, J.; Du, G.; Wu, H.; Gao, X.; Yang, Z.; Liu, B.; Cui, S. Protective effects of quercetin on traumatic brain injury induced inflammation and oxidative stress in cortex through activating Nrf2/HO-1 pathway. Restor. Neurol. Neurosci. 2021, 39, 73–84.
- Guan, Y.; Wang, J.; Wu, X.; Song, L.; Wang, Y.; Gong, M.; Li, B. Quercetin reverses chronic unpredictable mild stress-induced depression-like behavior in vivo by involving nuclear factor-E2-related factor 2. Brain Res. 2021, 1772, 147661.
- Yang, R.; Shen, Y.J.; Chen, M.; Zhao, J.Y.; Chen, S.H.; Zhang, W.; Song, J.K.; Li, L.; Du, G.H. Quercetin attenuates ischemia reperfusion injury by protecting the blood-brain barrier through Sirt1 in MCAO rats. J. Asian Nat. Prod. Res. 2022, 24, 278–289.
- Dai, W.; Wang, H.; Fang, J.; Zhu, Y.; Zhou, J.; Wang, X.; Zhou, Y.; Zhou, M. Curcumin provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE signaling pathway. Brain Res. Bull. 2018, 140, 65–71.
- Dong, W.; Yang, B.; Wang, L.; Li, B.; Guo, X.; Zhang, M.; Jiang, Z.; Fu, J.; Pi, J.; Guan, D.; et al. Curcumin plays neuroprotective roles against traumatic brain injury partly via Nrf2 signaling. Toxicol. Appl. Pharmacol. 2018, 346, 28–36.
- Liao, D.; Lv, C.; Cao, L.; Yao, D.; Wu, Y.; Long, M.; Liu, N.; Jiang, P. Curcumin Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behaviors via Restoring Changes in Oxidative Stress and the Activation of Nrf2 Signaling Pathway in Rats. Oxid. Med. Cell Longev. 2020, 2020, 9268083.
- Santana-Martínez, R.A.; Silva-Islas, C.A.; Fernández-Orihuela, Y.Y.; Barrera-Oviedo, D.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Maldonado, P.D. The Therapeutic Effect of Curcumin in Quinolinic Acid-Induced Neurotoxicity in Rats is Associated with BDNF, ERK1/2, Nrf2, and Antioxidant Enzymes. Antioxidants 2019, 8, 388.
- Wu, S.; Rao, G.; Wang, R.; Pang, Q.; Zhang, X.; Huang, R.; Li, T.; Tang, Z.; Hu, L. The neuroprotective effect of curcumin against ATO triggered neurotoxicity through Nrf2 and NF-κB signaling pathway in the brain of ducks. Ecotoxicol. Environ. Saf. 2021, 228, 112965.
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Muñoz-Ollero, P.; Forbes-Hernández, T.Y.; Esteban-Muñoz, A.; Giampieri, F.; Delgado Noya, I.; Bullón, P.; Vera-Ramírez, L.; et al. An Olive-Derived Extract 20% Rich in Hydroxytyrosol Prevents β-Amyloid Aggregation and Oxidative Stress, Two Features of Alzheimer Disease, via SKN-1/NRF2 and HSP-16.2 in Caenorhabditis elegans. Antioxidants 2022, 11, 629.
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin Confers Neuroprotection by Regulating Nrf2/TLR4/NF-κB Signaling in an Aβ Mouse Model. Mol. Neurobiol. 2019, 56, 6293–6309.
- Luo, Y.; Cui, H.X.; Jia, A.; Jia, S.S.; Yuan, K. The Protective Effect of the Total Flavonoids of Abelmoschus esculentus L. Flowers on Transient Cerebral Ischemia-Reperfusion Injury Is due to Activation of the Nrf2-ARE Pathway. Oxid. Med. Cell Longev. 2018, 2018, 8987173.
- Deng, Y.; Zhang, X.; Chen, F.; Huang, J.; Zhang, D.; Luo, J. HO-1 mediated by PI3K/Akt/Nrf2 signaling pathway is involved in (-)-epigallocatechin-3-gallate-rescueing impaired cognitive function induced by chronic cerebral hypoperfusion in rat model. Exp. Aging. Res. 2022, 48, 428–443.
- Xu, J.; Zhou, L.; Weng, Q.; Xiao, L.; Li, Q. Curcumin analogues attenuate Aβ25-35-induced oxidative stress in PC12 cells via Keap1/Nrf2/HO-1 signaling pathways. Chem. Biol. Interact. 2019, 305, 171–179.
- Liao, L.; Shi, J.; Jiang, C.; Zhang, L.; Feng, L.; Liu, J.; Zhang, J. Activation of anti-oxidant of curcumin pyrazole derivatives through preservation of mitochondria function and Nrf2 signaling pathway. Neurochem. Int. 2019, 125, 82–90.
- Huang, T.; Zhao, J.; Guo, D.; Pang, H.; Zhao, Y.; Song, J. Curcumin mitigates axonal injury and neuronal cell apoptosis through the PERK/Nrf2 signaling pathway following diffuse axonal injury. Neuroreport 2018, 29, 661–677.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-κB Signaling. Nutrients 2019, 11, 648.
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39.
