Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Michael Otto.
Staphylococcus aureus is a leading global pathogen that causes a considerable and hard-to-estimate number of moderately severe skin infections, but also more severe and sometimes fatal infections of the blood, bones, and lungs. The accessory gene regulator (Agr) system is undoubtedly the best-studied staphylococcal regulatory system, both in terms of its quorum-sensing mechanism and regarding its regulon and mechanisms of target gene control. It consists of an operon of four genes, agrB, agrD, agrC, and agrA, which form the quorum-sensing circuit.
- Staphylococcus aureus
- anti-virulence
- quorum-sensing
- quorum-quenching
1. Introduction
Staphylococcus aureus is a leading global pathogen that causes a considerable and hard-to-estimate number of moderately severe skin infections, but also more severe and sometimes fatal infections of the blood, bones, and lungs [1]. Most of the latter occur in the hospital, and despite a recent drop in S. aureus hospital-associated infections, S. aureus remains one of the biggest threats to immune-compromised patients, those undergoing surgery, or those with any kind of indwelling medical device. In the U.S., the fatality rate due to S. aureus sepsis alone has been at ~20,000 deaths annually in recent years [2]. Furthermore, widespread resistance to some of the best available anti-staphylococcal agents, such as penicillins and methicillin (methicillin-resistant S. aureus, MRSA), significantly increases the mortality, morbidity, and costs due to S. aureus infections [3].
With antimicrobial resistance on a steady rise, there is great interest in finding alternatives to antibiotics. These alternatives include, for example, vaccines, phage therapy, and anti-virulence approaches. For Gram-positive bacteria such as S. aureus, the situation may not be as dire as for some recently developed pan-resistant Gram-negative bacteria. Nevertheless, any non-antibiotic-based drug with therapeutic potential by itself or in combination with anti-staphylococcal antibiotics would be of great clinical use to combat S. aureus infections. This is especially true for those caused by MRSA, because the antibiotics to which MRSA remains susceptible are by far not as efficient as methicillin. Phage therapy is controversial for many reasons not to be discussed here; and despite great efforts over many years, there is no working vaccine for S. aureus [4][5]. Anti-virulence approaches have therefore been the focus of pre-clinical research aiming to find alternatives to antibiotics to treat S. aureus infections [6].
Many efforts have been taken to counter the effect of alpha-toxin and the many leukotoxins of S. aureus with monoclonal antibodies [7], but results are still outstanding or trials have been dropped probably due to early sobering results. Other anti-virulence strategies against S. aureus are all still in the pre-clinical stage. They comprise specific drugs against selected S. aureus virulence factors, such as the S. aureus pigment, staphyloxanthine [6][8]. Due to the fact that virtually all toxins and many other virulence factors of S. aureus are controlled by the quorum-sensing system Agr (which stands for accessory gene regulator) [9][10], much effort has been put into so-called quorum-quenching approaches, i.e., strategies to inhibit quorum-sensing control of S. aureus [11][12][13]. Of note, there is no other established quorum-sensing system of S. aureus than Agr. Claims of alleged quorum-sensing control by the so-called “RNAIII-inhibiting peptide” (RIP)/“Target of RAP”(TRAP) system was not supported by further studies [14][15] and whether the LuxS system, often claimed to be a “universal” quorum-sensing system, has any but a metabolic role in S. aureus remains uncertain [16].
 An interesting feature of the Agr system is that the different subgroups produce AIPs that inhibit the Agr circuit of other subgroups, except in rare cases when the AIPs are very similar (subgroups 1 and 4) [20]. Similar cross-inhibiting activity is generally observed for AIPs from other staphylococcal species, such as S. epidermidis [26][27].
An interesting feature of the Agr system is that the different subgroups produce AIPs that inhibit the Agr circuit of other subgroups, except in rare cases when the AIPs are very similar (subgroups 1 and 4) [20]. Similar cross-inhibiting activity is generally observed for AIPs from other staphylococcal species, such as S. epidermidis [26][27].
2. Quorum Sensing in Staphylococcus aureus: The Agr System
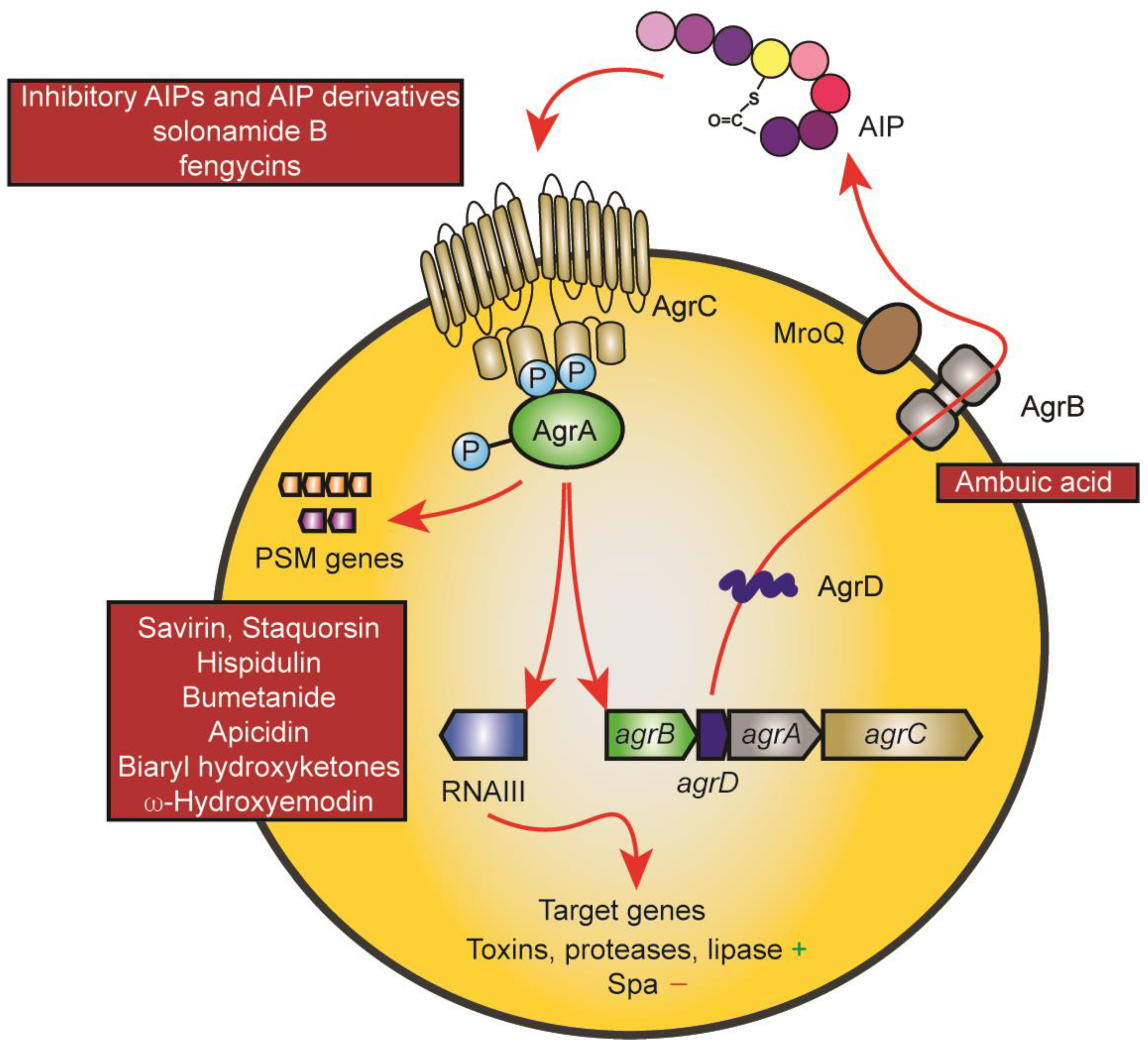
The Agr system is undoubtedly the best-studied staphylococcal regulatory system, both in terms of its quorum-sensing mechanism and regarding its regulon and mechanisms of target gene control [17][18][19]. It consists of an operon of four genes, agrB, agrD, agrC, and agrA, which form the quorum-sensing circuit (Figure 1). They are encoded adjacent to a regulatory RNA that is transcribed in the opposite direction and which is responsible for the control of most of the Agr regulon [17]. AgrB is the enzyme that post-translationally modifies the gene product of the agrD gene to form a thiolactone ring. Four different subgroups of Agr exist with considerable differences in the amino acid sequence of, and to some extent, length of the mature autoinducing peptide (AIP) [20]. The AIP can be a hepta- to nonapeptide which is exported likely also by AgrB and trimmed at the N-terminus with the help of the non-Agr-encoded membrane-located protease MroQ, at least for the AIPs of groups 1, 2, and likely 4, while the proteolytic maturation of the product of the AgrB thiolactone-introducing step remains unknown for subgroup 3 [21][22]. The AIP activates the AgrC-AgrA two-component system by binding to the membrane histidine kinase AgrC, which in turn phosphorylates AgrA, a DNA-binding response regulator that when phosphorylated binds to and activates the promoters driving transcription of agrBDCA and RNAIII [17]. AgrA also directly binds to the promoters of phenol-soluble modulin (PSM) genes, which are under exceptionally direct Agr regulation in contrast to other Agr-regulated genes that are controlled via RNAIII [23]. The characteristic phenotype of quorum-sensing is established by an initially low activity of the agrBDCA locus, which is strongly enhanced by the quorum-sensing feedback loop upon the accumulation of the AIP in a densely grown bacterial culture. The main biological purpose of this control in S. aureus is assumed to consist in withholding the production of toxins and secreted aggressive enzymes until the bacterial infection has grown to a stage when nutrients become scarce and tissue degradation is needed, and when the bacteria can withstand the inflammatory defensive mechanisms of the host that are triggered by these factors [17][18]. Interestingly, recent research also has established an essential role for Agr in the colonization of the skin and the intestine, for which the biological purpose remains to be investigated [24][25].
Figure 1. The Agr system and points of drug interference. The mechanisms underlying the Agr quorum-sensing circuit and target gene control are shown using red arrows. The AgrD AIP precursor is modified and exported by AgrB and further proteolytically trimmed by MroQ. Upon reaching a threshold concentration, the AIP leads to auto-phosphorylation of the membrane AIP sensor histidine kinase AgrC, which then leads to phosphorylation of the response regulator protein AgrA. AgrA promotes transcription from the promoters of the Agr system (agrBDCA promoter) and of the promoter controlling transcription of RNAIII, a regulatory small RNA that controls Agr target gene expression. PSM genes are under exceptional, direct control by AgrA promoter binding. Main points of attack by drugs are the AIP-AgrC interaction (top left) and AgrA and its interaction with target promoters (bottom left). The only drug targeting another mechanism is ambuic acid, which has been reported to interfere with AIP biosynthesis (AgrB).
3. Impact of Agr Control on S. aureus Infection and Colonization
The significant contribution of Agr to the progression of S. aureus infection has been demonstrated in several animal models, including infective endocarditis [28], skin and soft tissue infections including atopic dermatitis [9][25][29], pneumonia [30][31][32], and septic arthritis [33] and osteomyelitis [34]. These results are in good accordance with Agr control of major players that have been shown to drive these infections such as alpha-toxin, leukotoxins, and PSMs [35][36][37]. Contrastingly, Agr has the opposite effect on infections that are chronic and involve biofilms, such as infections of indwelling medical devices, prosthetic joint infections, or cystic fibrosis [18][38][39][40]. It also, somewhat paradoxically, promotes persistence in osteomyelitis [41]. This is often due to its exceptionally strict control of PSMs, which structure biofilms and lead to biofilm dispersal [42], and facilitate escape from host cells [43]. In the absence of PSMs in Agr dysfunctional mutants, biofilms grow thicker and more compact, which leads to increased resistance to leukocyte attacks and antibiotics [39][40]. In many host cells, the absence of Agr-controlled factors, including PSMs, leads to intercellular persistence [44][45]. The natural occurrence of Agr dysfunctional mutants has been observed for a long time and recently, their rise could be directly linked to the persistence of biofilm infections on indwelling devices [40]. Likely as a result of biofilm involvement in many cases of S. aureus bacteremia, which often originate from infections of indwelling devices, Agr dysfunctional strains are frequently isolated from S. aureus bacteremia [46] and Agr dysfunctionality has been shown to be associated with the unfavorable outcome of invasive S. aureus infection [47]. In rabbit models of indwelling medical device-associated versus non-device-associated infection, isogenic mutants in agr were less virulent in the former, but more virulent in the latter, in agreement with the human clinical results [48]. It was also interesting that in that study, Agr had a stronger impact on virulence in the non-device-associated model in mice than in rabbits. It was proposed that this may be due to the relatively increased impact of PSMs on infection outcome in mice, as they are not sensitive to many of the S. aureus leukotoxins, and to the fact that due to direct control by AgrA, Agr control of PSMs is much stronger than that of other S. aureus toxins [48]. Thus, while there has been much initial euphoria about targeting Agr for drug development, clinical findings and animal experiments have shown more recently that such use may be limited to specific types of infection. In particular, the potential exacerbation of chronic infection as well as device-associated bacteremia and potentially other invasive infections due to the impact on biofilms represent an important caveat. Furthermore, the impact of Agr on acute virulence may be exaggerated in some mouse models. More recently, Agr has also been implicated in non-symptomatic colonization by S. aureus [24][25]. From a therapeutic point of view, this is of importance as only about one- third of the human population is colonized by S. aureus, and colonization is associated with susceptibility to infection (as shown for nasal and intestinal S. aureus colonization) [49][50]. While no data appear to be available for the nose, potentially indicating that efforts to demonstrate a role of Agr in nasal colonization in animal models have failed, recent research indicates a role of Agr in skin and intestinal colonization [24][25] that may be employed for the purpose of decolonization and prevention of infection.References
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661.
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections-United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219.
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218.
- Miller, L.S.; Fowler, V.G.; Shukla, S.K.; Rose, W.E.; Proctor, R.A. Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 2020, 44, 123–153.
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus aureus Vaccine Research and Development: The Past, Present and Future, Including Novel Therapeutic Strategies. Front. Immunol. 2021, 12, 705360.
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different drugs for bad bugs: Antivirulence strategies in the age of antibiotic resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471.
- Cheung, G.Y.; Otto, M. The potential use of toxin antibodies as a strategy for controlling acute Staphylococcus aureus infections. Expert Opin. Ther. Targets 2012, 16, 601–612.
- Liu, C.I.; Liu, G.Y.; Song, Y.; Yin, F.; Hensler, M.E.; Jeng, W.Y.; Nizet, V.; Wang, A.H.; Oldfield, E. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science 2008, 319, 1391–1394.
- Cheung, G.Y.; Wang, R.; Khan, B.A.; Sturdevant, D.E.; Otto, M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 2011, 79, 1927–1935.
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569.
- Piewngam, P.; Chiou, J.; Chatterjee, P.; Otto, M. Alternative approaches to treat bacterial infections: Targeting quorum-sensing. Expert Rev. Anti-Infect. Ther. 2020, 18, 499–510.
- Khan, B.A.; Yeh, A.J.; Cheung, G.Y.; Otto, M. Investigational therapies targeting quorum-sensing for the treatment of Staphylococcus aureus infections. Expert Opin. Investig. Drugs 2015, 24, 689–704.
- Gray, B.; Hall, P.; Gresham, H. Targeting agr- and agr-like quorum sensing systems for development of common therapeutics to treat multiple gram-positive bacterial infections. Sensors 2013, 13, 5130–5166.
- Novick, R.P.; Ross, H.F.; Figueiredo, A.M.; Abramocjkin, G.; Muir, T.W. Activation and inhibition of the staphylococcal agr system. Science 2000, 287, 391a.
- Otto, M. Quorum-sensing control in Staphylococci–a target for antimicrobial drug therapy? FEMS Microbiol. Lett. 2004, 241, 135–141.
- Rezzonico, F.; Duffy, B. Lack of genomic evidence of AI-2 receptors suggests a non-quorum sensing role for luxS in most bacteria. BMC Microbiol. 2008, 8, 154.
- Novick, R.P.; Geisinger, E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008, 42, 541–564.
- Le, K.Y.; Otto, M. Quorum-sensing regulation in staphylococci-an overview. Front. Microbiol. 2015, 6, 1174.
- Wang, B.; Muir, T.W. Regulation of Virulence in Staphylococcus aureus: Molecular Mechanisms and Remaining Puzzles. Cell Chem. Biol. 2016, 23, 214–224.
- Ji, G.; Beavis, R.; Novick, R.P. Bacterial interference caused by autoinducing peptide variants. Science 1997, 276, 2027–2030.
- Stock, M.R.; Fang, L.; Johnson, K.R.; Cosgriff, C.; Teoh, W.P.; Alonzo, F., 3rd. Characterization of MroQ-Dependent Maturation and Export of the Staphylococcus aureus Accessory Gene Regulatory System Autoinducing Peptide. Infect. Immun. 2022, 90, e0026322.
- Zhao, A.; Bodine, S.P.; Xie, Q.; Wang, B.; Ram, G.; Novick, R.P.; Muir, T.W. Reconstitution of the S. aureus agr quorum sensing pathway reveals a direct role for the integral membrane protease MroQ in pheromone biosynthesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2202661119.
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.H.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-independent target gene control by the agr quorum-sensing system: Insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158.
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537.
- Nakamura, Y.; Takahashi, H.; Takaya, A.; Inoue, Y.; Katayama, Y.; Kusuya, Y.; Shoji, T.; Takada, S.; Nakagawa, S.; Oguma, R.; et al. Staphylococcus Agr virulence is critical for epidermal colonization and associates with atopic dermatitis development. Sci. Transl. Med. 2020, 12, eaay4068.
- Otto, M.; Sussmuth, R.; Vuong, C.; Jung, G.; Gotz, F. Inhibition of virulence factor expression in Staphylococcus aureus by the Staphylococcus epidermidis agr pheromone and derivatives. FEBS Lett. 1999, 450, 257–262.
- Olson, M.E.; Todd, D.A.; Schaeffer, C.R.; Paharik, A.E.; Van Dyke, M.J.; Buttner, H.; Dunman, P.M.; Rohde, H.; Cech, N.B.; Fey, P.D.; et al. Staphylococcus epidermidis agr quorum-sensing system: Signal identification, cross talk, and importance in colonization. J. Bacteriol 2014, 196, 3482–3493.
- Cheung, A.L.; Eberhardt, K.J.; Chung, E.; Yeaman, M.R.; Sullam, P.M.; Ramos, M.; Bayer, A.S. Diminished virulence of a sar-/agr- mutant of Staphylococcus aureus in the rabbit model of endocarditis. J. Clin. Investig. 1994, 94, 1815–1822.
- Wright, J.S., 3rd; Jin, R.; Novick, R.P. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc. Natl. Acad. Sci. USA 2005, 102, 1691–1696.
- Heyer, G.; Saba, S.; Adamo, R.; Rush, W.; Soong, G.; Cheung, A.; Prince, A. Staphylococcus aureus agr and sarA functions are required for invasive infection but not inflammatory responses in the lung. Infect. Immun. 2002, 70, 127–133.
- Bubeck Wardenburg, J.; Patel, R.J.; Schneewind, O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect. Immun. 2007, 75, 1040–1044.
- Montgomery, C.P.; Boyle-Vavra, S.; Daum, R.S. Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS ONE 2010, 5, e15177.
- Abdelnour, A.; Arvidson, S.; Bremell, T.; Ryden, C.; Tarkowski, A. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect. Immun. 1993, 61, 3879–3885.
- Gillaspy, A.F.; Hickmon, S.G.; Skinner, R.A.; Thomas, J.R.; Nelson, C.L.; Smeltzer, M.S. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 1995, 63, 3373–3380.
- Berube, B.J.; Bubeck Wardenburg, J. Staphylococcus aureus alpha-toxin: Nearly a century of intrigue. Toxins 2013, 5, 1140–1166.
- Alonzo, F., 3rd; Torres, V.J. Bacterial survival amidst an immune onslaught: The contribution of the Staphylococcus aureus leukotoxins. PLoS Pathog. 2013, 9, e1003143.
- Peschel, A.; Otto, M. Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 2013, 11, 667–673.
- Goerke, C.; Wolz, C. Adaptation of Staphylococcus aureus to the cystic fibrosis lung. Int. J. Med. Microbiol. 2010, 300, 520–525.
- He, L.; Le, K.Y.; Khan, B.A.; Nguyen, T.H.; Hunt, R.L.; Bae, J.S.; Kabat, J.; Zheng, Y.; Cheung, G.Y.C.; Li, M.; et al. Resistance to leukocytes ties benefits of quorum sensing dysfunctionality to biofilm infection. Nat. Microbiol. 2019, 4, 1114–1119.
- He, L.; Zhang, F.; Jian, Y.; Lv, H.; Hamushan, M.; Liu, J.; Liu, Y.; Wang, H.; Tang, J.; Han, P.; et al. Key role of quorum-sensing mutations in the development of Staphylococcus aureus clinical device-associated infection. Clin. Transl. Med. 2022, 12, e801.
- Butrico, C.E.; Cassat, J.E. Quorum Sensing and Toxin Production in Staphylococcus aureus Osteomyelitis: Pathogenesis and Paradox. Toxins 2020, 12, 516.
- Otto, M. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013, 64, 175–188.
- Surewaard, B.G.; de Haas, C.J.; Vervoort, F.; Rigby, K.M.; DeLeo, F.R.; Otto, M.; van Strijp, J.A.; Nijland, R. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol. 2013, 15, 1427–1437.
- Siegmund, A.; Afzal, M.A.; Tetzlaff, F.; Keinhorster, D.; Gratani, F.; Paprotka, K.; Westermann, M.; Nietzsche, S.; Wolz, C.; Fraunholz, M.; et al. Intracellular persistence of Staphylococcus aureus in endothelial cells is promoted by the absence of phenol-soluble modulins. Virulence 2021, 12, 1186–1198.
- Strobel, M.; Pfortner, H.; Tuchscherr, L.; Volker, U.; Schmidt, F.; Kramko, N.; Schnittler, H.J.; Fraunholz, M.J.; Loffler, B.; Peters, G.; et al. Post-invasion events after infection with Staphylococcus aureus are strongly dependent on both the host cell type and the infecting S. aureus strain. Clin. Microbiol. Infect. 2016, 22, 799–809.
- Fowler, V.G., Jr.; Sakoulas, G.; McIntyre, L.M.; Meka, V.G.; Arbeit, R.D.; Cabell, C.H.; Stryjewski, M.E.; Eliopoulos, G.M.; Reller, L.B.; Corey, G.R.; et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J. Infect. Dis. 2004, 190, 1140–1149.
- Lee, S.O.; Lee, S.; Lee, J.E.; Song, K.H.; Kang, C.K.; Wi, Y.M.; San-Juan, R.; Lopez-Cortes, L.E.; Lacoma, A.; Prat, C.; et al. Dysfunctional accessory gene regulator (agr) as a prognostic factor in invasive Staphylococcus aureus infection: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 20697.
- Cheung, G.Y.C.; Bae, J.S.; Liu, R.; Hunt, R.L.; Zheng, Y.; Otto, M. Bacterial virulence plays a crucial role in MRSA sepsis. PLoS Pathog. 2021, 17, e1009369.
- von Eiff, C.; Becker, K.; Machka, K.; Stammer, H.; Peters, G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 2001, 344, 11–16.
- Squier, C.; Rihs, J.D.; Risa, K.J.; Sagnimeni, A.; Wagener, M.M.; Stout, J.; Muder, R.R.; Singh, N. Staphylococcus aureus rectal carriage and its association with infections in patients in a surgical intensive care unit and a liver transplant unit. Infect. Control Hosp. Epidemiol. 2002, 23, 495–501.
More
