Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Xiaodong Wang.
Due to the global warming and dynamic changes in pathogenic virulence, leaf rust caused by Puccinia triticina has greatly expanded its epidermic region and become a severe threat to global wheat production. Leaf rust, caused by biotrophic fungal pathogen Puccinia triticina Erikss., is one of the most wide-spread and severe diseases in wheat all over the world.
- Wheat Leaf Rust
- Resistance
- Genetics
1. Introduction
Leaf rust, caused by biotrophic fungal pathogen Puccinia triticina Erikss., is one of the most wide-spread and severe diseases in wheat all over the world [1]. The yield loss caused by leaf rust ranges from 5% to 20%, and reaches about 50% during epidemics [2]. Compared with other rust diseases such as stripe rust and stem rust, leaf rust adapts to a more moderate temperature (10–25 °C). However, due to the global warming, leaf rust has greatly expanded its epidermic region and advanced its occurrence period [3]. Generally, seedling plants of wheat are more vulnerable to rust diseases, and all of these changes have made leaf rust become a new threat to global wheat productions.
Rational application of genetic loci controlling wheat resistance to leaf rust in breeding practice is still the best choice for the disease control. Future application of wheat leaf rust resistance genes requires more efficient co-operations between wheat researchers and breeders. In recent years, many leaf rust resistance (Lr)/quantitative trait locus (QLr ) have been identified and linked molecular markers have been developed. Knowledge regarding the sources and distribution of leaf rust resistance genes are important for developing new wheat cultivars with resistance. Wild wheat relatives are still valuable sources of novel genetic loci carrying Lr/QLr. Resistant germplasms including introgression lines generated from wheat relatives remain to be explored. With recent progresses of sequenced genomes of wheat relatives/progenitors, more resistance genes are expected to be cloned from these sources [151][4]. Previous issues such as long breeding periods and drawback from unwanted chromosome introgression will be solved by potential linkage drag with advanced techniques in speed breeding [152][5].
2. Pathogenic Profile
Leaf rust has a complicated life cycle including asexual stage on wheat and sexual stage on alternative hosts (Figure 1a,b) [1]. At the asexual stage, leaf rust infects wheat plant via its urediospores (Figure 1a). Urediospores can spread over long distances with air flow and re-infect wheat plants multiple times. Since leaf rust has a broad host range including wheat, barley, and their wild relatives, it can easily over-summer on grasses and volunteer crops. On the other hand, it normally over-winter on wheat as latent hypha or urediospores. At the sexual stage, leaf rust produces telia on wheat leaves in the late growing season (Figure 1a); teliospores from telia further infects alternative hosts including Thalictrum spp. or Leptopyrum fumarioides; pycnia/pycniospores and aecium/aeciospores can be formed on the alternative hosts; fertilization occur between pycniospores and receptive hyphae with opposite mating type combinations; aeciospores infect back to the host plants of wheat and produce uredium/urediospores to complete the life cycle (Figure 1b).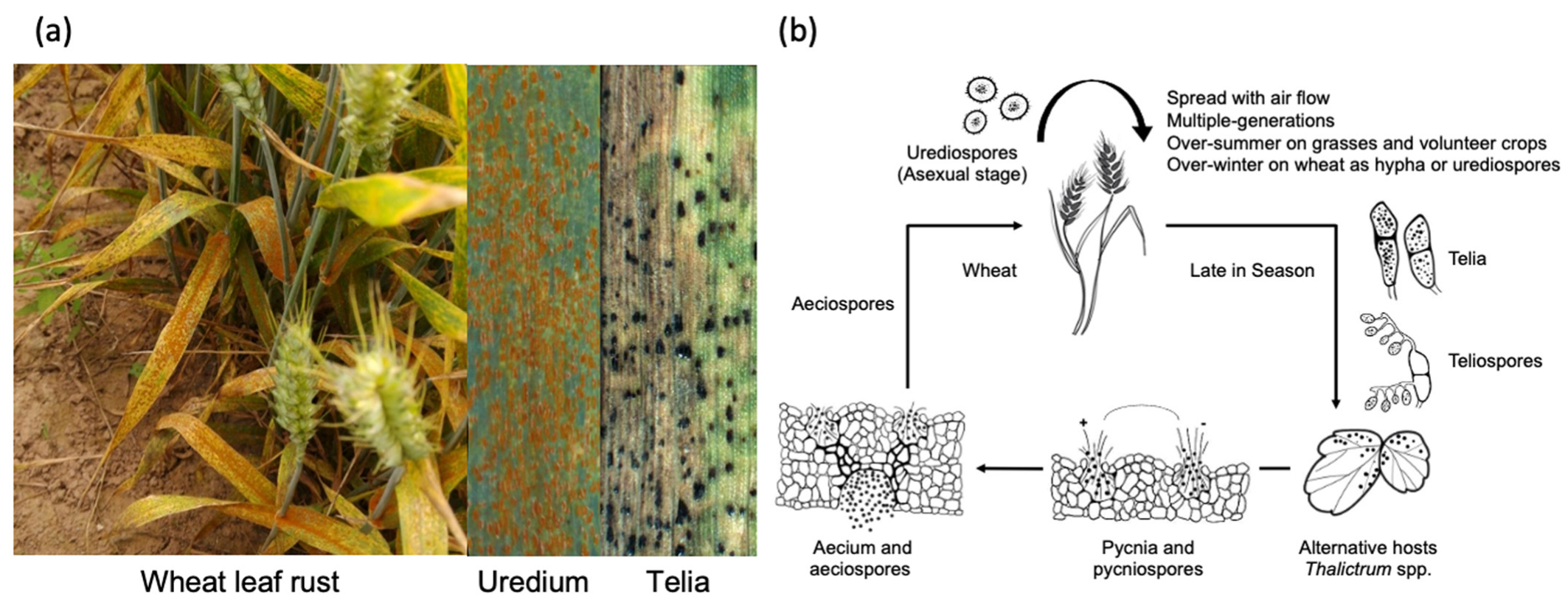
Figure 1. Pathogenic profile of Puccinia triticina. (a) Leaf rust infects wheat leaves via its urediospores at the asexual stage. Telia is produced on wheat leaves in the late growing season. (b) Life cycle of P. triticina can be divided into asexual stage on wheat and sexual stage on alternative hosts. Teliospores infect alternative hosts Thalictrum spp. and later produces pycnia and aecium. Aeciospores infect back to wheat plants to complete the life cycle.
3. Types of Wheat Resistance to Leaf Rust
Based on the physiological features, genetic determinations, and molecular mechanisms, wheat resistance to leaf rust can be classified into two types (Table 1): race-specific resistance and slow rusting resistance. The race-specific resistance follows the gene-for-gene theory. Currently, most of the cloned Lr genes controlling this type of resistance, including Lr1, Lr10, Lr13, Lr21, Lr22a, and Lr42, encode nucleotide binding site leucine-rich repeat (NBS-LRR) proteins [4,5,6,7,8,9][6][7][8][9][10][11]. As modeled in Arabidopsis, upon directly or indirectly recognition of avirulence (Avr) proteins secreted by phytopathogens, NBS-LRR proteins form a homo-pentamer called resistosome, which penetrates the cell membrane of the responsive cells and eventually results in the observed hypersensitive responses (HR) or necrosis on wheat leaves [10][12]. A recent protein crystallization study on wheat stem rust resistance protein Sr35 and its corresponding avirulent protein AvrSr35 revealed a similar resistosome structure [11][13]. Besides these NBS-LRR proteins, another race-specific resistance gene, Lr14a, encodes a membrane-localized protein containing multiple ankyrin repeats and Ca2+ channels [12][14]. The other recently cloned high-resistant gene Lr9/Lr58 encodes a tandem kinase fusion protein [13][15]. Notably, certain race-specific resistance genes are functioning only at seedling stage but lost their resistance against multiple Pt pathotypes in the field at the adult plant stage. Others may keep their high resistance to leaf rust at the adult plant stage as hypersensitive adult plant resistance (APR) or all-stage (AS) race-specific resistance.Table 1.
Types of wheat resistance to leaf rust.
| Type of Resistance | Resistance Stage | Resistant Features | Cloned Resistance Genes |
|---|---|---|---|
| Race-specific resistance | Seedling stage | Seedling resistance. Immune or hypersensitive response (cell death/necrosis) observed on the leaf surface. May be lost at adult plant stage against multiple Pt pathotypes in the field. | Lr1, Lr10, Lr13, Lr21, Lr22a, Lr42 (NBS-LRR) [4,65,][76,][87,]8,9][[9][10][11] Lr14a (Ankyrin repeats and Ca2+ channels) [12][14] Lr9/Lr58 (Tandem kinase) [13][15] |
| Adult plant stage | Hypersensitive adult plant resistance (APR)/All-stage (AS) race-specific resistance. Immune or hypersensitive response (cell death/necrosis) observed on the leaf surface. | ||
| Slow rusting | Adult plant stage | Non-race-specific resistance. A lower level but more durable resistance. Rust infection and sporulation can be accomplished in a much delayed and reduced manner. | Lr34 (ATP-binding cassette transporter) [14][16] Lr67 (Hexose transporter) [15][17] |
References
- Bolton, M.D.; Kolmer, J.A.; Garvin, D.F. Wheat leaf rust caused by Puccinia triticina. Mol. Plant Pathol. 2008, 9, 563–575.
- Eversmeyer, M.; Kramer, C. Epidemiology of wheat leaf and stem rust in the central great plains of the USA. Annu. Rev. Phytopathol. 2000, 38, 491.
- Helfer, S. Rust fungi and global change. New Phytol. 2014, 201, 770–780.
- Wulff, B.B.; Krattinger, S.G. The long road to engineering durable disease resistance in wheat. Curr. Opin. Biotech. 2022, 73, 270–275.
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29.
- Cloutier, S.; McCallum, B.D.; Loutre, C.; Banks, T.W.; Wicker, T.; Feuillet, C.; Keller, B.; Jordan, M.C. Leaf rust resistance gene Lr1, isolated from bread wheat (Triticum aestivum L.) is a member of the large psr567 gene family. Plant Mol. Biol. 2007, 65, 93–106.
- Feuillet, C.; Travella, S.; Stein, N.; Albar, L.; Nublat, A.; Keller, B. Map-based isolation of the leaf rust disease resistance gene Lr10 from the hexaploid wheat (Triticum aestivum L.) genome. Proc. Natl. Acad. Sci. USA 2003, 100, 15253–15258.
- Yan, X.; Li, M.; Zhang, P.; Yin, G.; Zhang, H.; Gebrewahid, T.W.; Zhang, J.; Dong, L.; Liu, D.; Liu, Z. High-temperature wheat leaf rust resistance gene Lr13 exhibits pleiotropic effects on hybrid necrosis. Mol. Plant 2021, 14, 1029–1032.
- Huang, L.; Brooks, S.A.; Li, W.; Fellers, J.P.; Trick, H.N.; Gill, B.S. Map-based cloning of leaf rust resistance gene Lr21 from the large and polyploid genome of bread wheat. Genetics 2003, 164, 655–664.
- Thind, A.K.; Wicker, T.; Šimková, H.; Fossati, D.; Moullet, O.; Brabant, C.; Vrána, J.; Doležel, J.; Krattinger, S.G. Rapid cloning of genes in hexaploid wheat using cultivar-specific long-range chromosome assembly. Nat. Biotech. 2017, 35, 793–796.
- Lin, G.; Chen, H.; Tian, B.; Sehgal, S.K.; Singh, L.; Xie, J.; Rawat, N.; Juliana, P.; Singh, N.; Shrestha, S. Cloning of the broadly effective wheat leaf rust resistance gene Lr42 transferred from Aegilops tauschii. Nat. Commun. 2022, 13, 3044.
- Wang, J.; Hu, M.; Wang, J.; Qi, J.; Han, Z.; Wang, G.; Qi, Y.; Wang, H.-W.; Zhou, J.-M.; Chai, J. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 2019, 364, eaav5870.
- Zhao, Y.-B.; Liu, M.-X.; Chen, T.-T.; Ma, X.; Li, Z.-K.; Zheng, Z.; Zheng, S.-R.; Chen, L.; Li, Y.-Z.; Tang, L.-R.; et al. Pathogen effector AvrSr35 triggers Sr35 resistosome assembly via a direct recognition mechanism. Sci. Adv. 2022, 8, eabq5108.
- Kolodziej, M.C.; Singla, J.; Sánchez-Martín, J.; Zbinden, H.; Šimková, H.; Karafiátová, M.; Doležel, J.; Gronnier, J.; Poretti, M.; Glauser, G. A membrane-bound ankyrin repeat protein confers race-specific leaf rust disease resistance in wheat. Nat. Commun. 2021, 12, 956.
- Wang, Y.; Abrouk, M.; Gourdoupis, S.; Koo, D.-H.; Karafiátová, M.; Molnár, I.; Doležel, J.; Athiyannan, N.; Cavalet-Giorsa, E.; Jaremko, Ł.; et al. An unusual tandem kinase fusion protein confers leaf rust resistance in wheat. Res. Sq. 2022. preprint.
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-Espino, J.; McFadden, H.; Bossolini, E.; Selter, L.L.; Keller, B. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 2009, 323, 1360–1363.
- Moore, J.W.; Herrera-Foessel, S.; Lan, C.; Schnippenkoetter, W.; Ayliffe, M.; Huerta-Espino, J.; Lillemo, M.; Viccars, L.; Milne, R.; Periyannan, S. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 2015, 47, 1494–1498.
- Hatta, M.A.M.; Steuernagel, B.; Wulff, B.B. Rapid gene cloning in wheat. In Applications of Genetic and Genomic Research in Cereals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 65–95.
More
