Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Gianfranco Pintus and Version 3 by Jessie Wu.
Adverse pregnancy outcomes are considered significant health risks for pregnant women and their offspring during pregnancy and throughout their lifespan. Maternal dietary intake of polyphenolic antioxidants is linked to a reduced risk of maternal obesity and cardio-metabolic disorders, positively affecting both the fetus and offspring. TIn this work, we will gather and critically appraise the current literature highlighting the effect/s of the naturally occurring polyphenol antioxidant resveratrol on oxidative stress, inflammation, and other molecular and physiological phenomena associated with pregnancy and pregnancy conditions are discussed, such as gestational diabetes, preeclampsia, and preterm labor.
- resveratrol
- pregnancy
- placenta
1. Resveratrol’s Mechanism of Action during Pregnancy Complications
Interestingly, maternal resveratrol intake has been reported to have potential beneficial effects in adverse human pregnancies [1][3] and has been intensively studied in rodent models as a potential therapeutic agent in several pregnancy-related disorders, including pre-eclampsia (PE) [2][42], gestational diabetes mellitus (GDM) [3][22], fetal-growth restriction (FGR) [4][43], insulin resistance, and dyslipidemia [5][6][23,44]. However, the molecular mechanisms underlying its efficiency are not entirely understood [7][45]. Studies suggested that resveratrol’s potential protective mechanisms in adverse pregnancy outcomes are related to its pleiotropic properties, including antioxidant, anti-inflammatory, anti-obesogenic, anti-atherosclerotic, and anti-diabetic, which evidently associate its consumption with the prevention of several non-communicable diseases [8][9][20,46]. Figure 1 schematizes the potential molecular mechanisms that may underpin the protective effects of resveratrol intake in mothers and their offspring in different models.
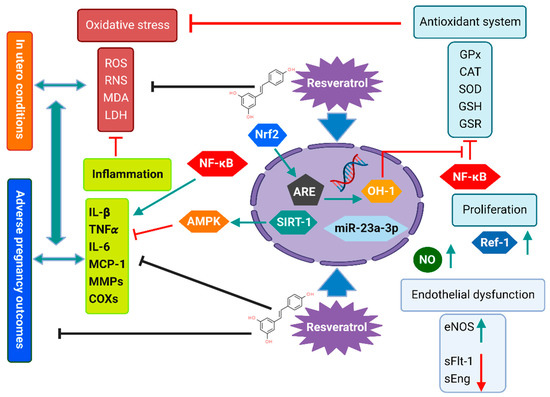
Figure 1.
Potential molecular mechanisms underpinning the effects of resveratrol intake in mothers and their offspring in different models.
A systematic data analysis across different species and dosages indicated that resveratrol consumption could decrease inflammation and oxidative stress in placental and embryonic tissues [1][3]. Oxidative stress is a phenomenon caused by increased levels of reactive oxygen species (ROS) resulting in disturbance of the cellular redox balance, which causes the impairment of cellular functions and eventually initiates pathological pathways leading to disease development [10][11][47,48]. It is now evident that oxidative stress is significantly higher during normal pregnancy compared to non-pregnancy periods in women [12][49]. In fact, the gestational and prenatal changes of the maternal body, as well as the high oxygen and energy required for fetal development, favor ROS overproduction [13][50]. These elevated ROS levels appear to be implicated in pregnancy-related disorders such as PE, intrauterine growth restriction (IUGR), and fetal death [14][15][51,52]. Thus, evaluating the redox status during pregnancy in order to find adequate strategies to counteract it appears to be an issue of utmost importance.
In this regard, studies in murine models have shown that oxidative stress biomarkers and metabolic dysfunction caused by a low-protein diet in the mother, placenta and offspring could be improved by a resveratrol-rich diet in pregnant individuals [16][53]. Anti-atherogenic activities, low fetal oxidative stress, and reduced apoptosis were also detected in rodent streptozotocin-induced diabetic models treated with resveratrol [17][18][54,55]. Moreover, increased risk of infertility is often linked to advanced maternal age, where the major oxidative stress-associated causative factors encompass decreased oocyte quality, low fertilization rate, poor embryonic development, low pregnancy rate, and a high rate of chromosomal aberrations, telomerase shortening, and apoptosis [19][20][56,57]. In this regard, by delaying cells’ aging process and preventing age-related diseases through ameliorating mitochondrial function and reducing ROS generation, several studies have reported that resveratrol supplementation can improve in vitro oocyte maturation and embryonic developmental competence in different species [21][22][23][24][58,59,60,61]. A study realized in a pregnant-aged mice model demonstrated that resveratrol intake increases blastocyst development, ameliorates pregnancy and implantation rates, and parallelly decreases ROS production with a significant increase in mitochondrial membrane potential, suggesting that resveratrol supplementation may improve pregnancy oxidative stress-mediated outcomes in women with advanced maternal age [25][62]. In this regard, Liu et al. [26][63] have also suggested that resveratrol can induce oocyte maturation and subsequent embryonic development in aged mice [26][63]. Indeed, aging-associated reproductive pathologies are frequently associated with impaired DNA repair, metabolic disorders, genomic instability, telomeric shortening, apoptosis, and mitochondrial dysfunction [21][27][28][58,64,65], which are the molecular target of resveratrol action.
Epigenetic regulation represents another potential mechanism related to the maternal resveratrol intake effects and modulates methylation and acetylation processes affect gene expression [29][66]. Among the main epigenetic changes exerted by resveratrol in the zygotic pronuclei are the methylation and acetylation of histone H3 on lysine 9 (H3K9). Gestational resveratrol supplementation was demonstrated to induce breast cancer-1 promoter (BRCA-1) hypermethylation and to decrease BRCA-1 expression in the mammary tissue of rat offspring [8][20]. Another mechanism through which resveratrol’s maternal intake may improve pregnancy outcomes is by relieving oxidative stress-related mitophagy [30][31][67,68]. Mitophagy is defined as the selective removal of dysfunctional mitochondria by autophagy, a process aberrantly affected by ROS increase and physiologically restored by oxidative stress balance [32][69]. Consonantly, a study by Zha et al. [33][70] demonstrated that resveratrol supplementation in pregnant mice might promote mammary gland proliferation and antioxidant activity through mitophagy activation [33][70]. Pregnancy-related metabolic disorders, such as GDM, occur when ROS prevents insulin from facilitating cellular glucose uptake, subsequently leading to insulin resistance [34][71]. Hypertensive disorders of pregnancy comprising chronic, white, mask and gestational hypertension, PE, hemolysis, elevated liver enzymes, and low platelet count were also found to be tightly related to altered pro-inflammatory and oxidative stress signals and be responsible for the increased risk of chronic diseases such as obesity, type 2 diabetes, and cardiovascular diseases in adult life [8][35][36][37][20,72,73,74].
Resveratrol is also recognized as a Sirtuin 1 (SIRT1) activator. The Sirtuin proteins family has a beneficial impact on longevity mainly linked to their effects on metabolic control since they are substantially contributing to lipid and glucose regulation via the deacetylation of crucial metabolic signals relevant to the activated.
The Sirtuin proteins family has a beneficial impact on longevity primarily related to their effects on metabolic control as they substantially contribute to lipid and glucose regulation through the deacetylation of crucial metabolic signals related to the activated protein kinase-SIRT1-PPARG coactivator-1α axis [38][39][75,76]. In this context, a study realized on rats exposed to a maternal high-fat diet during pregnancy, resveratrol supplementation (10 mg/kg) managed to restore the impaired expression of SIRT1, phosphor-extracellular regulated kinases1/2, (p-ERK1/2) and phospho-peroxisome proliferator-activated receptor γ (PPARγ), adiponectin and Brain-Derived Neurotrophic Factor (BDNF), all molecules involved in insulin resistance and mild cognitive dysfunction [40][77]. These previous data underline resveratrol’s ability to improve many maternal-associated complications including PE, FGR, diabetes, insulin resistance (IR), obesity, and metabolic syndrome [40][77].
2. Maternal Pregnancy-Related Disorders
2.1. Resveratrol Effect in Pre-Eclampsia and Related Disorders
Pregnancy is known to cause increased levels of oxidative stress, as an aspect of the systemic inflammatory response, resulting in high-circulated amounts of ROS, with the placenta representing the main source [41][78]. PE is a pregnancy-specific clinical syndrome characterized by a multifactorial physiopathology [42][43][79,80], that includes both new-onset of hypertension and new-onset proteinuria ≥300 mg/24 h after 20 weeks of gestation, most often near-term. However, when proteinuria registers normal levels, PE is considered thrombocytopenia-related hypertension, with altered liver function, renal insufficiency, pulmonary oedema, and new-onset cerebral or visual disturbances [44][81]. One condition that features oxidative stress during pregnancy is hypoxia, a common form of intrauterine stress defined as an abnormal exposure to low oxygen levels, resulting in an excessive generation of ROS [45][82]. Accordingly, increased placental hypoxia and uterine vascular resistance were revealed to be associated with PE and fetal/intrauterine growth restriction (FGR/IUGR) in several animal and human studies [46][83]. Gestational hypoxia induces ROS overproduction in uteroplacental cells’ mitochondria leading to oxidative stress [46][83]. In contrast, excessive ROS production causes uteroplacental dysfunction by altering cellular macromolecules, which underlies PE and FGR pathogenesis [46][83]. Resveratrol has been recently shown to exert a protective effect against prenatal hypoxia-induced programming of the metabolic syndrome when offspring-initiated treatment at weaning [47][84]. In a recent study, resveratrol intake (4 g/kg) managed to ameliorate adverse fetal outcomes in a rat model of severe prenatal hypoxia [48][28].
PE-associate oxidative stress originates from the placental impairment caused by hypoxia-reoxygenation imbalance, lower efficiency of both free radical scavengers and antioxidant enzymes, impaired angiogenesis, decreased nitric oxide (NO) bioavailability vascular endothelial dysfunction, cardiovascular complications, and exaggerated inflammatory response [49][50][51][52][53][85,86,87,88,89]. A poorly perfused fetoplacental unit releases free radicals and initiates oxidative stress impairment in placental cells [52][54][55][88,90,91]. Resveratrol was demonstrated to activate endothelial nitric oxide synthase (eNOS) and increase NO and nuclear factor-erythroid-derived 2-related factor-2 (Nrf2) production [56][92]. In response to resveratrol, Nrf2 binds to the antioxidant response element (ARE) promoter upregulating the expression of antioxidant proteins, including heme oxygenase-1 (HO-1) and glutathione reductase (GSR), thus counteracting oxidative stress and balancing the cellular redox state [57][58][93,94]. Indeed, in an in vitro PE model, resveratrol increased ARE activity and reduced ROS in endothelial cells exposed to plasma from PE patients compared to cells exposed to plasma from healthy pregnancies. Noteworthy, plasma from PE patients obtained 1 hr after the ingestion of polyphenol-rich whole red grapefruit juice (rich in resveratrol) significantly increased NO production and reduced antioxidant markers in the exposed cells compared with the serum before juice intake [58][94].
PE etiology is not fully understood, however, this condition is believed to be associated with impaired uterine artery blood flow [59][95]. In this context, recent studies have shown that resveratrol can induce uterine arteries relaxation during PE pathological events. Interestingly, resveratrol supplementation with a dose of 4 g/kg was demonstrated to ameliorate the PE-like phenotype by significantly increasing uterine artery blood flow velocity and fetal weight in pregnant C57BL/6J, eNOS⁻/⁻, and COMT⁻/⁻ mice murine models, suggesting resveratrol consumption as a potential therapeutic strategy for PE disorder [59][95].
It has been recently shown that circulating SIRT1 is reduced during PE [60][96]. In this regard, a group of researchers investigated the effects of resveratrol as a SIRT1 activator using the previously reported in vitro/clinical PE model compared to gestational hypertensive (GH) and healthy pregnant (HP) women. The authors showed that resveratrol-elicited SIRT1 activation alone did not exert any effect and may not be beneficial to women with PE, suggesting that pregnant women with PE may have different responsive mechanisms to this molecule [60][96]. Furthermore, aberrant trophoblast invasion is among the factors involved in PE occurrence and development since it contributes to the progression of multiple conditions and is characterized by impaired spiral artery remodeling [61][97], cell-matrix restructuring, and cytoskeleton dynamics [62][98], during which epithelial-mesenchymal transition (EMT) occurs leading to the breakdown of cell–cell adhesion, the loss of epithelial phenotypes, and cell depolarization [63][64][99,100]. During pregnancy, the acquisition of invasive phenotypes by the extra-villous trophoblast (EVT), was found to be involved in PE pathogenesis and was suggested to be linked to EMT [65][101]. In this regard, using both in vitro (HTR-8/SVeno cell culture) and in vivo (NG-nitro-l-arginine methyl ester mice model) PE models, Zou et al. [66][102] suggested that resveratrol (100 µmol/L resveratrol /20 mg/kg/day) might stimulate the invasive capability of human trophoblasts by promoting a Wnt/β-catenin pathway-mediated EMT transformation, increasing trophoblasts migration and the invasion in the cell model, and markedly ameliorating hypertension and proteinuria in mice [66][102]. Molecularly, resveratrol-mediated EMT activation occurred through the regulation of E-cadherin, β-catenin, N-cadherin, and vimentin expression, and altered the WNT-related gene expression, including WNT1, WNT3, and WNT5B [66][102].
Several studies suggested that the resveratrol-associated anti-hypertensive effect is mainly related to its capacity to attenuate blood pressure and hypertension symptoms. Interestingly, a clinical trial with 400 PE patients investigated the outcome of a nifedipine/resveratrol combined treatment against PE, revealing that resveratrol supplementation significantly reduced the time needed to control blood pressure with a better result in the group treated with both nifedipine (10 mg, up to 5 dosages) and resveratrol, which also had a significantly delayed time between the hypertensive crises intercourse (50 mg, up to 5 dosages) [5][23]. In contrast, resveratrol did not produce any apparent effect on rats in the study by Moraloglu et al. [2][42], where a Desoxycorticosterone acetate (DOCA) hypertension PE model was used to test the potential hypotensive effect of resveratrol on the blood flow. Interestingly, DOCA managed to increase blood pressure and placental and renal blood flow levels, whereas, no significant differences for the same parameters were rated in groups treated with resveratrol (20 mg/kg per day) [2][42].
Consistent with this, other studies indicated that resveratrol anti-hypertensive properties are also related to its ability to inhibit the release of endothelial dysfunction-associated anti-angiogenic factors and that their elevation is a main player in placental oxidative stress and inflammation during the maternal endothelial dysfunction in PE [67][103]. For instance, serum soluble fms-like tyrosine kinase-1 (sFlt-1), also known as a soluble receptor for vascular endothelial factors (VEGF), is a protein that binds and decreases the concentrations of circulating VEGF and placental growth factor (PlGF) [68][69][104,105]. While Endoglin (Eng) is a transmembrane glycoprotein and is considered another factor playing a role in PE etiology and an accessory receptor for the transforming growth factor-beta (TGF-β). Eng is highly expressed in syncytiotrophoblast and proliferating endothelial cells [70][106], it influences the TGF-β and eNOS signaling pathways resulting in a significant angiogenesis modulation [71][107]. In this regard, a study carried out in vitro by Hannan et al. [72][108] demonstrated that resveratrol (0–100 μM) decreases sFlt-1 and soluble Eng secretion from primary trophoblasts and human umbilical vein endothelial cells (HUVECs), a phenomenon that positively correlated with increased mRNA expression of the pro-inflammatory molecules NFκB, IL-6, and IL-1β in the trophoblast and decreased of IL-6, IL-1β, and TNF-α [72][108]. Additionally, resveratrol significantly increased the mRNA expression of several antioxidant enzymes including HO-1, NADPH Quinone Dehydrogenase (1NQO1), Glutamate-Cysteine Ligase Catalytic (GCLC) subunit and thioredoxin (TXN) in HUVECs, while reducing HO-1 protein levels in trophoblast cells. Expression of the cell adhesion molecule VCAM-1 and the adhesion of peripheral blood mononuclear cells were also increased by resveratrol supplementation [72][108]. Further, TNF-α-induced endothelial dysfunction in HUVECs was significantly ameliorated by resveratrol through i) the reduction in TNF-α-induced Endothelin-1 (a vasoconstrictor) expression and ii) the increase in endothelial eNOS phosphorylation [72][108]. Resveratrol (20 mg/kg/day) has also been reported to prevent cells from p53- and ROS-dependent apoptosis induced by IL-1β in an NG-Nitro-l-arginine methyl ester-induced PE rats model via the increase in superoxide dismutase (SOD) suggesting that resveratrol significantly opposes oxidative stress effects in vivo [73][109]. Overall, resveratrol can be suggested to alleviate PE symptoms and decrease its effects in pregnant individuals and their offspring in different models via multiple pathways mainly related to the attenuation of vascular injury, placental dysfunction, hypertension symptoms, anti-angiogenic factors, oxidative stress and inflammatory responses Table 1 Taken together, the reported data suggests resveratrol as a potential therapeutic tool in PE.
Table 1.
Studies using resveratrol in the treatment of models of pre-eclampsia in pregnancy.
| Reference | Type of the Study | PE Experimental Model | Dose | Mechanism of Action | Outcomes of Supplementation in the Experimental Model |
|---|---|---|---|---|---|
| [58][94] | In vitro/Clinical trial | -ECs -Human |
200 mL of polyphenol rich-grape fruit/1 µM of trans-resveratrol | NO, HO-1, miRNA expression, GSH, ARE, ROS levels | ↑ ARE, NO, HO-1, GSH, ARE ↓ ROS, oxidative stress No studied effect in offspring |
| [60][96] | In vitro/Clinical trial | -ECs -Human |
200 mL of polyphenol rich-grape fruit/1µM of trans-resveratrol | SIRT1 expression | No significant effect No studied effect in offspring |
| [66][102] | In vitro/In vivo | -HTR-8/SVeno cell culture/ -NG-nitro-l-arginine methyl ester mice model (L-NAME) |
100 µmol/L resveratrol 20 mg/kg/day |
Expression of genes regulating migration, invasion, angiogenesis and EMT-related factors in trophoblasts Hypertension and proteinuria measurement. Endothelial dysfunction/ injury |
↑ E-cadherin, β-catenin, N-cadherin, and vimentin expression, ↓ Alteration of WNT-related gene expression, including WNT1, WNT3 and WNT5B. ↓ Hypertension and proteinuria No studied effect in offspring |
| [72][108] | In vitro | primary trophoblasts and HUVECs | 0–100 μM | -Angiogenesis activation (sFlt-1) (sEng) secretion Anti-inflammatory effect -NFκB , IL-6 and IL-1β -Antioxidant effect HO-1, NQO1, GCLC, (TXN) Endothelial dysfunction, VCAM Endothelin-1 eNOS |
↓ sFlt-1, sEng ↓ IL-6, IL-1β and TNF-α.‘ ↑, NQO1, GCLC TXN ↓ HO-1 protein in trophoblast. ↑ VCAM-1 ↓ Endotelin-1, ↑eNOS No studied effect in offspring |
| [73][109] | In vivo | NG-nitro-l-arginine methyl ester mice model (L-NAME) | 20 mg/kg/day | -Antioxidant effect, Apoptosis SOD, MDA |
↑ SOD, MDA No studied effect in offspring |
Ecs: Endothelial cells, NO: Nitric oxide, HO-1: Heme oxygenase-1, miRNA: microRNA, GSH: Gluthatione, ARE: antioxidant response element, ROS: Reactive oxygen species, SIRT1: sirtuin1, WNT: WNT genes, sFlt-1: soluble fms-like tyrosine kinase-1, sEng: soluble endoglin, NFκB: nuclear factor-kappa B, IL-6: Interleukin-6, IL-1β: Interleukin-1β, TXN: thioredoxin, NADPH, NQO1: NADPH Quinone Dehydrogenase 1, TNF-α: Tumor necrosis factor-alpha, GCLC: Glutamate-Cysteine Ligase Catalytic Subunit, VCAM-1: Vascular cell adhesion molecule-1, eNOS: endothelial nitric oxide
2.2. Resveratrol Effect in Gestational Diabetes and Related Metabolic Disorders
GDM is a type of diabetes characterized by an impaired glucose tolerance that occurs during pregnancy [74][110]. Although GDM is a reversible pathology and the impaired metabolism returns to normal after delivery, the risk of developing type 2 diabetes later is high [75][111]. GDM is extremely harmful to mothers and their offspring and may induce PE, premature rupture of membranes, and premature delivery [76][112]. The main features of GDM during pregnancy include increased glucose demand, increased insulin resistance, and relative insufficiency in insulin secretion [77][113]. Recent studies emphasized the protective effects of resveratrol in diabetes and its related-cardiovascular complications, involving the regulation of multiple signaling pathways, inhibition of oxidative stress and inflammation, enhancement of insulin sensitivity, induction of autophagy, regulation of lipid metabolism, promotion of GLUT4 expression, translocation, and activation of SIRT1/AMPK signaling axis [78][114]. Several studies have demonstrated that resveratrol lowers maternal blood sugar levels, ameliorates the maternal lipid profile [79][115], and prevents delays in embryo development in rat diabetic dams models [17][54]. In a recent study performed in streptozotocin GDM pregnant rat model, the ability of resveratrol to lower blood glucose and blood lipids was tested. Resveratrol supplementation demonstrated a dose-dependent effect (120 and 240 mg/kg), which positively correlates with a decrease in both insulin and blood glucose levels in comparison with the control group; remarkedly demonstrating a stronger effect than metformin hydrochloride in improving GDM outcomes [80][116]. Using the same previous model, it was reported that the resveratrol-zinc oxide complex encapsulated with chitosan (CS-ZnO-RS) (an encapsulated form of resveratrol to increase the stability and effectiveness of substances in gestational diabetes management), significantly decreased the blood glucose levels and maintained the lipid content similar to control group levels, while simultaneously reducing the level of pro-inflammatory factors (IL-6 and MCP-1) and significantly decreasing endoplasmic reticulum stress components (GRP78, p-IRE1α, p-eIF2α, and p-PERK), and further inhibiting α-glucosidase and α-amylase activity in a dose-dependent fashion [81][117].
Studies also demonstrated that GDM is often related to adverse metabolic health outcomes in offspring as a common pregnancy complication. In a GDM model of female Sprague Dawley rats fed with a high-fat and sucrose diet, Brawerman et al. [82][118] showed that maternal resveratrol supplementation (147 mg/kg/day) protects against GDM-induced glucose intolerance and offspring islet dysfunction by restoring glucose tolerance and normoglycemia and improving insulin secretion. At 15 weeks of age, hepatic steatosis, IR, glucose intolerance, and dysregulated gluconeogenesis were attenuated in offspring of resveratrol-treated dams [82][118]. Moreover, the dysregulation of several metabolic genes (e.g., lpl: lipoprotein lipase; ppara: peroxisome proliferator-activated receptor α; g6p: glucose-6-phosphatase) were also attenuated whilst glucose-stimulated insulin secretion was improved in the offspring islets of resveratrol-treated dams [82][118].
It is well known that insulin resistance (IR) caused by insufficient insulin secretion during pregnancy, may lead to metabolic disorders, mainly GDM and related pathologies [83][119]. Attention was recently given to the role of resveratrol in ameliorating IR in pregnancy-related metabolic disorders. Consistently, recent studies have shown that resveratrol may improve glucose uptake in adipocytes with IR, ameliorate IR in mice fed with a high-fat diet, and enhances glucose metabolism and insulin tolerance in GDM mice (100 mg/kg) [84][85][120,121]. MicroRNAs (miRNAs), a type of short-chain non-coding endogenous RNAs existing in eukaryotic organisms, are known to exert regulatory effects on several cell functions and play a different role in many diseases [86][87][88][89][90][122,123,124,125,126]. Recent studies confirmed the resveratrol’s capacity to regulate their expression [91][16], especially that of MiR-23a-3p, which was reported to be low-expressed in adipose tissues of obese and diabetic patients; in fact, its over-expression can reduce TNF-α-induced IR in adipocytes [92][127]. In a study realized on a high-fat diet GDM mice model, resveratrol supplementation (0.2%) managed to ameliorate glucose uptake and lipid metabolism, regulating the miR-23a-3p/NOV axis through the increase in Adiponectin, Leptin, Phosphoinositide 3-kinase (p-PI3K), and phosphorylated Akt (p-Akt) in adipocytes with IR and parallelly decreasing the nephroblastoma (NOV) overexpression [93][128]. In diabetic embryopathy-murine models, resveratrol (50 mg, up to 5 dosages) significantly ameliorates the embryonic outcome in terms of diminishing developmental abnormalities, a phenomenon likely associated with its antioxidative potential, anti-diabetic action, and anti-dyslipidemic nature [5][23].
Maternal obesity is also a metabolic complication that rates high maternal and fetal oxidative stress and inflammation. Hypothetically, offspring of obese mothers are at greater risk of developing obesity, diabetes, and cardiovascular disorders in adult life [94][129], with risk factors that include variations in both maternal glucose and lipid metabolism (predisposition to GDM in particular), abnormal pregnancy hormone concentrations [95][96][97][130,131,132], stillbirth, premature birth, and macrosomia [97][98][132,133].
A recent study conducted in obesity murine models revealed that maternal resveratrol supplementation (20 mg/kg/day) improves maternal metabolism and reduces the placental and liver oxidative stress of mothers and fetuses in a sex-dependent manner [99][134]. Compared to the untreated group, resveratrol supplementation in pregnant animals was able to lower adipocytes number, triglycerides serum concentrations, insulin resistance, liver fat accumulation, expression of genes related to insulin resistance, inflammatory processes, and lowered oxidative stress in mothers, placentas, and female fetal liver [99][134]. Resveratrol treatment (5 mg/kg/day) was shown to improve some of the altered metabolic symptoms in a programmed prenatal and postnatal high-fat exposure in the progeny of Sprague Dawley dams’ experimental model, including peripheral leptin resistance, and related dysbiosis of the gut [100][101][135,136]. Another study suggested that resveratrol’s protective effects during pregnancy and lactation are diet dependent, where resveratrol supplementation (2.0 to 2.5 mg/kg/d/dam) decreased body weight and adipose tissue content in offspring of dams on a high-fat diet but did not affect offspring from the low-fat diet-fed dams [102][137]. In addition, resveratrol supplementation (300 mg/kg) was demonstrated to increase high-density lipoprotein cholesterol and low-density lipoprotein cholesterol in the plasma and partially improved the fat metabolism in the adipose tissue in piglets’ experimental model [103][138]. Intrahepatic cholestasis of pregnancy (ICP), is a pregnancy-specific liver disease characterized by raised serum bile acids and adverse fetal outcomes partially related to SIRT1 dysregulation [104][139]. Liao et al. [104][139] studied the molecular and biochemical mechanism underpinning resveratrol’s regulation of the SIRT1- nuclear factor-κB (SIRT1-NF-κB) signaling pathway and bile acid biosynthesis in ICP. Resveratrol (30–120 mg/kg/day) was demonstrated to decrease bile acid levels in the ICP rat model and was suggested to protect syncytiotrophoblast against trichloroanisole (TCA)-induced inflammatory injury through the upregulation of SIRT1 and the downregulation of RelA/p65, a subunit of NF-κB recognized as an activator of SIRT1 [104][139]. In a related study, it was shown that resveratrol ameliorates ICP conditions by downregulating the overexpression of matrix metalloproteinases (MMPs) [105][140]. Indeed, in the ethinylestradiol-induced ICP rat model, resveratrol-diet (15 mg/kg/day) was able to inhibit the elevation of both MMP-2 and MMP-9, and exhibited better outcomes in restoring bile flow rate, serum enzymatic activities, and total bile acids (TBA) concentration compared to the ICP known drug ursodeoxycholic acid [105][140].
On the other hand, diabetes-associated lipid profile disruption may indirectly affect embryogenesis and organogenesis [106][141], and the impairment of the couple glucose levels/lipid status may simultaneously alter the fetal and neonatal growth; which may later impact offspring life and contribute to adult obesity. Using the same previous GDM model, Singh et al. [3][22] evaluated the effect of resveratrol on lipidic profile variations. Compared with the untreated GDM group, resveratrol supplementation demonstrated a dose-dependent effect (120 and 240 mg/kg), which significantly increased both HDL and adiponectin levels and inversely correlated with the levels of leptin, resistin, TNF-α, IL-6 levels, the body weight, total cholesterol (TC), triglycerides (TG), and low-density lipoprotein (LDL) [3][22]. Parallelly, resveratrol was shown to reduce mRNA levels of 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA reductase), the rate-limiting enzyme of cholesterol synthesis and the statin drugs target in high-fat fed hamster model [107][142]. Statin is an active drug that is used to lower human cholesterol and triglycerides but may be harmful to pregnant or nursing women [108][143]; therefore, in light of these findings, the authors suggested resveratrol as a potential therapy for an altered lipidic profile during pregnancy-associated metabolic disorders.
Overall, the above-mentioned studies evaluated the short-term effects of maternal resveratrol intake in diabetic and related-metabolic disorders experimental models Table 2. Resveratrol showed favorable results on metabolic homeostasis and redox state. Nevertheless, the mother and offspring’s longer-term outcomes remain poorly specified. Not to mention the variations of experimental models, range of doses, routes of administration and therapeutic periods that may contribute to inconclusive results regarding the potential metabolic benefits of maternal resveratrol supplementation during pregnancy and lactation. In this light, extended studies with well-established experimental models are needed.
Table 2.
Studies using resveratrol in the treatment of models of gestational diabetes miletus (GDM) in pregnancy.
| Reference | Type of the Study | GDM Experimental Model | Dose | Mechanism of Action | Outcomes of Supplementation in the Experimental Model |
|---|---|---|---|---|---|
| [80][116] | In vivo | Streptozotocin GDM pregnant rats model | 120 and 240 mg/kg | Amelioration of blood glucose and blood lipids levels | ↑ Insulin levels, ↓ blood glucose levels Amelioration of lipidic profile No studied effect in offspring |
| [81][117] | In vivo | Streptozotocin GDM pregnant rats model | 500 μg/mL (CS-ZnO-RS) | Anti-diabetic effect Anti-inflammatory effect |
↓ blood glucose levels lipid content reduced the level of ↓ IL-6 and MCP-1, GRP78, p-IRE1α, p-eIF2α, and p-PERK Inhibition of α-glucosidase and α-amylase No studied effect in offspring |
| [3][22] | In vivo | Streptozotocin GDM pregnant rats model | 120 and 240 mg/kg | Amelioration of the lipids metabolic profile | ↑ HDL-C and adiponectin ↓ leptin, resistin, TNF-α, IL-6 levels, the body weight, TC, TG, and LDL-C No studied effect in offspring |
| [107][142] | In vivo | high-fat fed hamster model | 0.025% | Amelioration of the lipids metabolic profile | -HMG-CoA reductase expression No studied effect in offspring |
| [93][128] | In vivo | high-fat diet GDM mice model IR adipocyte model was established by dexamethasone-inducing |
0.2% | Amelioration of glucose and lipids metabolic profile Amelioration of IR in adipocytes |
↓ The bodyweight, serum glucose ↑ serum insulin Upregulations of miR-23a-3p/NOV axis ↑ Adiponectin, Leptin, p-PI3K, and p-Akt No studied effect in offspring |
| [82][118] | In vivo | female Sprague-Dawley rat model, fed with a high-fat and sucrose diet | 147 mg/kg/day | Protection against gestational diabetes-induced glucose intolerance and islet dysfunction | -Restored glucose tolerance, normoglycaemia and improved insulin secretion in offspring -Attenuation of hepatic steatosis, insulin resistance, glucose intolerance and dysregulated gluconeogenesis in offspring -Downregulation of lpl, ppara, g6p genes |
| [109][144] | In vivo | C57BL/KsJ-Lep (db/+) (db/+) genetic GDM pregnant mouse model | 10 mg/kg/day | Amelioration of glucose metabolic profile via SIRT1/AMPK pathway Amelioration of IR |
↑ Glucose metabolism, insulin tolerance and reproductive outcome of the pregnant db/+ females AMPK activation ↓ glucose-6-phosphatase in both pregnant db/+ females and their offspring |
GDM: Gestational diabetes miletus, CS-ZnO-RS: resveratrol-zinc oxide complex encapsulated with chitosan, IL-6: Interleukin-6, MCP-1: Monocyte Chemoattractant Protein-1, GRP78, p-IRE1α, p-eIF2α, and p-PERK: endoplasmic reticulum stress components, TNF-α: Tumor necrosis factor-alpha, TC: Total cholesterol, TG: Triglycerides, LDL-C: low-density lipoprotein-C, IR: Insulin resistance, miR-23a-3p/NOV axis: microRNA-23a-3p, p-Akt: phosphorylated-AKT, p-PI3K: Phosphoinositide 3-kinase, lpl: lipoprotein lipase gene, ppara: Peroxisome proliferator-activated receptor alpha, g6p: glucose-6 phosphatase. AMPK: AMP-activated protein kinase.
