Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jue Li and Version 3 by Conner Chen.
Unsaturated soil is a form of natural soil whose pores are filled by air and water. Different from saturated soil, the microstructure of unsaturated soil consists of three phases, namely, the solid phase (soil particle), vapor phase, and liquid phase. Due to the matric suction of soil pores, the hydraulic and mechanical behaviors of unsaturated soils present a significant dependence on the moisture condition, which usually results in a series of unpredictable risks, including foundation settlement, landslide, and dam collapse. Microbial-induced calcite precipitation (MICP) is a novel and environmentally friendly technology that can improve the water stability of unsaturated soft or expansive soils.
- microbial-induced calcite precipitation
- unsaturated soil
- reaction mechanism
- influencing facors
- MICP
1. Introduction
Unsaturated soil is a type of soil consisting mainly of solid particles, a liquid matrix, and pore air. Due to the shrinkage interface between liquid and air, the matrix suction becomes an important structural stress within saturated soil [1]. The mechanical properties of unsaturated soil closely depend on the humidity characteristics [2][3][2,3]. The dependence of mechanical behaviors on matric suction or moisture involved in many engineering problems has been reported on in studies of foundation engineering, subgrade engineering, and slope engineering [4][5][6][7][8][4,5,6,7,8]. For example, after the soft soil humidifying, the stiffness of the soil subgrades decreased evidently, resulting in the settlement and collapse of the pavement structure. Thus, unsaturated soils should be improved in practice by physical and chemical methods.
The existing soil treatments mainly include dynamic compaction methods and the cement grouting method, but these methods all have certain shortcomings [9][10][9,10]. Yao et al. [11] strengthened the collapsible dam foundation by means of the dynamic compaction method, and found that its availability of treatment is limited by construction and geological conditions. The grouting method is often used for soft foundation treatment, but it uses a large amount of cement and its production would increase the quantity of CO2 emissions [12]. More and more attention has been placed on reducing CO2 emissions in the life cycle of engineering practices, with the increasing awareness of people concerned about the environment [13]. These green and low-carbon practices require a novel technology to decouple the dependence of the soil’s treatment by cement. Therefore, the microbial-induced calcite precipitation (MICP) technology has become one of the interest points in geotechnical and geological engineering fields in recent years, since it is an environmentally friendly, noiseless, and low-cost approach for improving the engineering properties of unsaturated soil [14][15][14,15].
At present, the cement process of MICP could mainly be classified into four types of bio-mineralization reactions [16], including the urea hydrolysis type [17], ferric reduction type [18], sulfate reduction type [19] and denitrification type [20]. Urea hydrolysis is the most efficient and advantageous way to conduct MICP technology, since it has the advantages of simple operation and is easily controllable [21]. In addition, it can quickly produce calcium carbonate precipitates and has a high microbial survival rate without a special nutrient solution [22]. Studies have been conducted to investigate the improvement of soil mechanical behaviors by urea hydrolysis through macroscopic and microcosmic experiments [23][24][23,24]. Martinez et al. [25] applied the MICP grouting method on expansive soil columns and found that the swelling potential and hydrophilicity of expansive soil decreased after the treatment of MICP. Salifu et al. [26] demonstrated that the penetration grouting approach of MICP-solidified fluid can improve the stability of soil slopes, since the generated CaCO3 can fill the volume of the micro-pore structures within soil by 9.9%. Sharma and R. [27] found that the compressive strength of MICP-treated soil was 1.45 to 2.26 times of that of the untreated soil by laboratory tests. Some researchers have reported the effectiveness of MICP treatment on soil mechanical properties, while less considerations and comparisons have been made on the changes of the soil hydraulic properties induced by MICP treatment [28][29][30][28,29,30]. Specially, it is important to investigate the moisture dependence on the stiffness and deformation of unsaturated soil in subgrade construction.
2. Mechanism of Microbial-Induced Calcite Precipitation (MICP)
The MICP is a widely existing bio-mineralization reaction in nature, accompanied by different microbial activities and chemical processes [31]. Different from the mineralization on the geological surface of earth, bio-mineralization refers to the process in which inorganic elements selectively precipitate from the environment to form minerals on a specific organic matrix with the participation of biological cells. This bio driven mineralization reaction mainly occurs in four ways: urea hydrolysis, denitrification, sulfate reduction, and ferric iron reduction [32]. The method of using microorganism-induced denitrification to precipitate calcium carbonate not only has high cultivation cost, but also has low efficiency of generating calcium carbonate [33]. However, it is undeniable that denitrifying bacteria can grow in situ and play a role under anoxic conditions [34]. In the process of microbial-induced sulfate, the sulfate reduction will produce the hydrogen sulfide gas, which is harmful to the environment and human body [35]. In addition, hydrogen sulfide gas also results in accelerating the corrosion of steel bars in structures. For the ferric iron reduction method, the requirements for the oxidation substrate are very high, meaning that it only works when the solubility of the oxidation substrate is low [36]. The urea hydrolysis method is simple and efficient, given that there is no additional reaction condition and no environmental pollution [37]. Because matrixes (urea and CaCl2) have a high solubility in water solution, the MICP process can generate a lot of CaCO3 in a short time [38]. Scholars generally believe that MICP using urea hydrolysis has an enormous potential in soil treatment [39]. In addition, among all biochemical reactions, the reaction of urea hydrolysis is a main technological path to produce ammonium ions and carbonate ions, and its reaction process is relatively simple. Therefore, the reaction mechanism of urea hydrolysis MICP was taken as an example in the following section. Chuo et al. [40] found that 17–30% of bacteria collected from Australia can hydrolyze urea rapidly. The Bacillus pasteurii (BP) has a high urease activity within soil and has been widely used in the MICP treatment [41]. During its metabolism, its cell secretes a large amount of urease to produce adenosine triphosphate (ATP), which promotes the catalytic hydrolysis of urea to produce ammonium and carbonate ions. Meanwhile, the PH value in the system increases in this process. Due to the presence of calcium ions, carbonate ions and calcium ions gradually modulate to form CaCO3 precipitation. The reaction equation of urea hydrolysis is shown in Equations (1)–(5) [42].CO(NH2)2+H2O→NH2COOH+NH3
NH2COOH+H2O→NH3+H2CO3
H2CO3→2H++CO32−
NH3+H2O→NH4++OH−
Ca2++CO32−→CaCO3↓
CO(NH2)2+2H2O→H2CO3+2NH3(g)
H2CO3+2NH3↔2NH4++2OH−
H2CO3→H++HCO3−
HCO3−+H++2OH−↔CO32−+2H2O
Ca2++CO32−↔CaCO3(s)
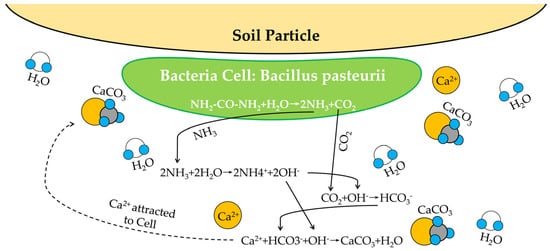
Figure 1.
Sketch map of microbial-induced carbonate deposition process on particle surface.
3. Influencing Factors of MICP Reaction
The essence of MICP technology is to induce microorganisms to generate CaCO3 precipitation between the gaps within soil to achieve the role of bio-cementation and treatment of soil. However, MICP technology will be limited and constrained by many factors in actual operation [46]. The factors that influence the treatment effect of MICP technology contain temperature, PH value, bio-cement concentration, calcium ion concentration, nutrient solution (urea) concentration, and soil particle size [47]. Mortensen et al. [48] showed that the influencing factors were in the order from large to small as temperature, concentration of bio-cementing fluid, nutrient solution concentration, PH value, and calcium ion concentration, respectively, through single factor and orthogonal tests. Sotoudehfar et al. [49] used the optimized orthogonal test method to explore the influence of various parameters in the process of MICP on the curing effect. The results showed that the curing time had the greatest influence on the curing effect, and the bacterial cell concentration, molar concentration ratio of nutrient solution, and the liquid injection flow rate had similar influences on the curing effect.3.1. Temperature and PH Value
Temperature is the key factor in the success of MICP technology on soil treatment [50]. A change in temperature will affect the growth of bacteria, the activity of microbial enzymes, the biodegradation of bacteria, and the process of binding precipitation, thus affecting the final curing effect. Figure 2a shows the changes of urease and growth activities of BP at different temperatures. An absorbance index at the 600 nm wavelength (OD600) was wildly applied to evaluate the density and growth activity of the BP solution. It found that the growth curve of BP was different when the temperature varied from low to high [51]. It is generally believed that the growth of BP is inhibited at low temperatures, while the urease activity of BP decreases at high temperatures [52][53][52,53]. Therefore, the BP should be cultivated under a suitable growth environment (temperature).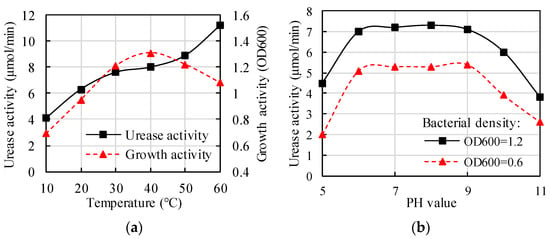
Figure 2.
Changes of urease activity influenced by (
a
) Temperature (
b
) PH value.
