Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Telma Encarnação.
Bioplastics are one of the possible alternative solutions to the polymers of petrochemical origins. Bioplastics have several advantages over traditional plastics in terms of low carbon footprint, energy efficiency, biodegradability and versatility.
- Bioplastics
- Biopolymers
- Conventional polymers
- Biodegradability
- Sustainability
- Renewable resources
1. Introduction
The use of polymeric materials is widely spread around the world. These materials have significant advantages compared with other, more conventional materials, such as metals and wood, mainly because of their properties and performance.
It is estimated that 99% of these polymeric materials come from fossil fuels. These plastics entail several issues since their primary raw material is a hazard to environment conservation [1].
The durability and degradability of these materials are two contradictory topics. For most applications, it is favourable that the material maintains specific properties throughout time, but it is also desirable to discard them easily after their use. There are some alternative processes usually used to manage this kind of waste: recycling (one of the most sustainable waste management processes but requires a controlled process to have a final product with good properties) and energy recovery (allows the production of energy by burning the waste but ends up producing toxic emissions and greenhouse gases) [2,3][2][3]. However, a massive quantity of material ends up in landfills or even abandoned, and some of it reaches the ocean. A long-term study took place on the North Atlantic Sea, where it was observed a seawater sample contained 580,000 pieces of plastic per square kilometre. This waste management has created a crisis, since landfills have a limited capacity, high costs and strict legislation [4].
The remaining percentage of plastics is produced from natural raw materials and are denominated bio-based plastics or bioplastics [1]. The use of bioplastics dates centuries ago. In 1500 BCE, Mesoamerican cultures (Maya, Aztecs) used natural rubber and latex to make containers and waterproof their clothes. However, only in 1862 was the first manmade bioplastic produced (Parkesine, a bioplastic made from cellulose), created by Alexander Parkes. The first company to produce bioplastics was Marlborough Biopolymers in 1983. They produced strips, filaments, chips, panels and powders of bacteria called Biopol. More recently, in 2018, Project Effective was launched with the goal of replacing nylon with bio-nylon, and it created the first bioplastic made from the fruit [5].
Although investigations regarding bioplastics have been done for over a century, their implementation and extensive production is not yet developed. Figure 1 presents a chart of the last few years’ production and the forecast for the years to come. In 2019, 1.95 Mt of bioplastic was produced, corresponding to about 0.6% of all plastic production worldwide.
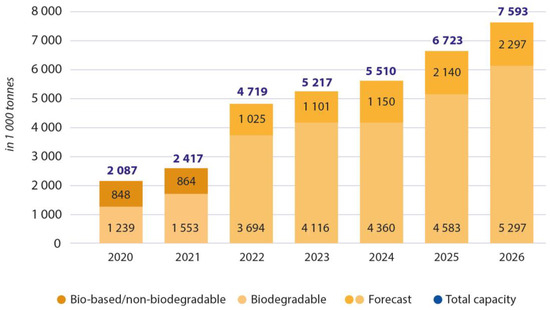
Figure 1. Global production capacities of bioplastics 2021–2026. Adapted from European Bioplastics, “Bioplastics Market Development Update 2021”. https://docs.european-bioplastics.org/publications/market_data/Report_Bioplastics_Market_Data_2021_short_version.pdf (accessed on 29 December 2022) [6].
The small production of these plastics is mainly due to their more expensive manufacturing and generally inferior mechanical properties compared to fossil-based polymers. However, it is necessary to develop these materials to have a sustainable alternative to petrochemical materials [7]. ThiRes paper earchers will discuss current scenarios and the inherent production limitations and present the pros and cons of producing and using bioplastics to replace some petrochemical-based polymers.
2. Bioplastics Composites
The synthetic assembly of two or more materials, a matrix binder and selected reinforcing agents, is used for various applications. The goal is to overcome the weaknesses and increase versatility. The most common fibres exercised in recent times are glass fibres, carbon fibres, aramid fibres, natural fibres, nylon and polyester fibres. There is a clear advantage to using natural-based fibres; for example, they are biodegradable, renewable, available in bulk, cheaper and lighter [54,55,56][8][9][10].2.1. Coating
Coating bioplastics is an excellent technique to improve some of the properties of these materials; it is specially used to enhance the barrier properties. By applying a thin layer of other polymers on top of the bioplastic, the tensile strength and elasticity can be improved, as well as increasing oxygen and water vapour permeability and resistance. Some examples of usually used coatings are listed below:-
PLA barrier properties (oxygen and water vapour) can be improved by applying a PLA-Si/SiOx, AlOx (aluminium oxide), PCL-Si/SiOx or PEO-Si/SiOx (polyethylene oxide) coating.
-
When coated with PLA, SPI (soy protein isolate) films tensile strength increases from 2.8 to 17.4 MPa, and the elongation went from 165.7% to 203.4%. However, the water vapor permeability decreases 20- to 60-fold, depending on the PLA concentration in the coating solution.
-
Nitrocellulose or PVdC (polyvinylidene chloride) coating on cellophane is also used to improve oxygen and water vapour barrier properties.
2.2. Nanocomposites
For a composite to be considered a nanocomposite, it must have at least one of its types of particles with dimensions in the nano range. The composite can be classified as polymer layered crystal nanocomposites (Figure 72a), nanotubes or whiskers (Figure 2b) and isodimensional nanoparticles (Figure 72c), according to the number of dimensions it has: three, two or one, respectively [2].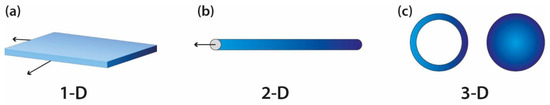
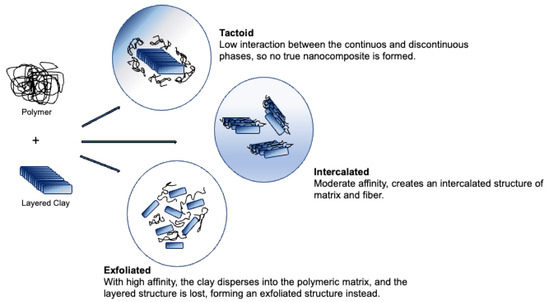
Figure 3. Structures of polymer nanoclay composite.

Figure 4. Different batches of sawdust used (a) (less than 0.7 mm), (b) (between 0.7 and 1.4 mm) and (c) (between 1.4 and 2.8 mm). From the project EcoPlast.
2.3. Cellulose
Adding cellulose to the bioplastic is another way to influence some properties of the final material. It is possible to have good adhesion between the fibre and the matrix due to the chemical similarity between starch and natural fibres. The main effect this addition has on the composite is the reduction of water vapour permeability due to the fibres’ highly crystalline and hydrophobic character, also affecting the Young’s modulus, tensile strength and the elongation break [8][11].References
- Plastic Soup. What Are Bioplastics? Available online: https://www.plasticsoupfoundation.org/en/plastic-problem/what-is-plastic/bioplastics/ (accessed on 2 October 2021).
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571.
- Rebeiz, K.S.; Craft, A.P. Plastic waste management in construction: Technological and institutional issues. Resour. Conserv. Recycl. 1995, 15, 245–257.
- Arikan, E.B.; Ozsoy, H.D. A Review: Investigation of Bioplastics. J. Civ. Eng. Arch. 2015, 9, 188–192.
- Bioplasticsnews. The History of Bioplastics. Available online: https://bioplasticsnews.com/2018/07/05/history-of-bioplastics/ (accessed on 15 October 2021).
- European Bioplastics. Bioplastics Market Development Update 2021. Available online: https://docs.european-bioplastics.org/publications/market_data/Report_Bioplastics_Market_Data_2021_short_version.pdf (accessed on 4 January 2022).
- Fredi, G.; Dorigato, A. Recycling of bioplastic waste: A review. Adv. Ind. Eng. Polym. Res. 2021, 4, 159–177.
- Lubin, G. Handbook of Composites; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013.
- Prashanth, S.; Subbaya, K.M.; Nithin, K.; Sachhidananda, S. Fiber Reinforced Composites—A Review. J. Mater. Sci. Eng. 2017, 6, 3.
- Chong, T.Y.; Law, M.C.; Chan, Y.S. The Potentials of Corn Waste Lignocellulosic Fibre as an Improved Reinforced Bioplastic Composites. J. Polym. Environ. 2021, 29, 363–381.
- Peelman, N.; Ragaert, R.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141.
- Kortaberria, G.; Tercjak, A. Block Copolymer Nanocomposites, 1st ed.; Jenny Stanford Publishing: New York, NY, USA, 2016.
More
