Ten percent to fifteen percent of the global population suffers from osteoarthritis (OA), a condition characterized by cartilage deterioration and synovitis. It is a rapidly developing modern disease
[1].
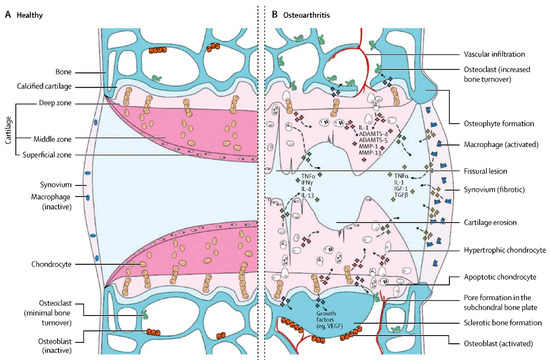
Figure 1 summarizes the pathological process in detail
[2]. There are many risk factors, such as sex, age, trauma, obesity and genetics. Since the middle of the last century, OA has not only shown its highest worldwide incidence rate
[3] but has also forced various developed countries to bear heavy economic burdens because it is a crippling disease
[4][5][4,5]. Currently, there is no ideal specific drug used for the clinical treatment of OA. To relieve inflammation and discomfort in early stages, nonsteroidal anti-inflammatory medications and analgesics, such as glucosamine, chondroitin supplements, and intra-articular local injections of corticosteroids are primarily employed
[6]. Though it is an intrusive procedure that is frequently accompanied by significant uncontrolled complications, joint replacement plays a prominent role in advanced cases
[7][8][7,8]. However, the service life of the prosthesis and the functional recovery of the damaged limbs are not as positive as
rwe
searchers originally anticipated and can even be described as restricted
[6]. As a result, research into a more secure and efficient treatment for OA is crucial.
2. Characteristics of MSCs for the OA Therapy
MSCs, as specific types of adult stem cells, possess great potential in bone tissue engineering and regenerative therapy due to their capacity for self-renewal and differentiation
[19][20][21][19,20,21]. The multipotency, wide availability, and low immunogenicity of MSCs have made them a hot topic in the bioremediation field. In 1976, Friedenstein et al.
[22] identified and prepared MSCs from different tissues, including fat, placenta, umbilical cord, synovium, bone, and dental pulp
[23]. As shown in
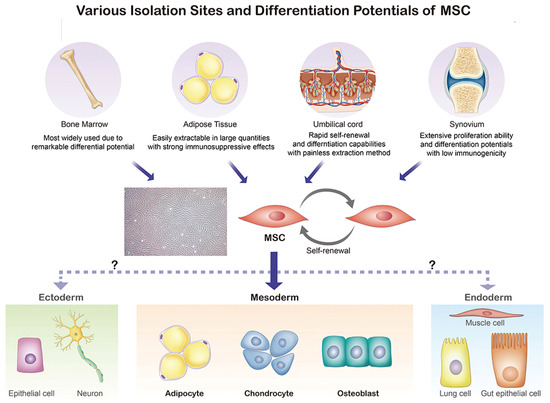
Figure 2, MSCs, which are mainly extracted from bone marrow, adipose tissue, umbilical cord, and the synovium, possess the ability to differentiate into adipocytes, chondrocytes, and osteoblasts.
Figure 2. Origin and differentiation directions of MSCs. MSC, mesenchymal stem cell.
The International Society for Cell Therapy established three criteria for defining MSCs. First and foremost, they exhibit adhesion growth characteristics under conventional culture conditions. Secondly, MSCs attached to culture plastic must display significant levels of CD105, CD73, and CD90, but not CD45, CD34, CD14, CD11b, CD79a, CD19, and HLADR surface markers after being detected with flow cytometry. Thirdly, MSCs can differentiate into bone-forming cells (chondrocytes and osteoblasts) or fat-storing cells (adipocytes) when cultivated in a certain induction media
[24][25].
In general, the proliferative capacity of MSCs obtained from fetal tissue is more outstanding than that of MSCs obtained from adult tissue; additionally, umbilical cord, amniotic membrane, and fat sources are more accessible and possess a higher proliferative capacity, while the proliferation ability of bone marrow-derived MSCs (BM-MSCs) is relatively inadequate
[25][26][26,27]. MSCs isolated from adipose tissue are the most genetically and morphologically stable, and they proliferate best over an extended period of incubation
[27][28]. Gene expression analysis revealed that BM-MSCs express higher levels of osteogenic differentiation-related genes than MSCs isolated from umbilical cords
[28][29][29,30]. Nevertheless, compared with BM-MSCs, umbilical cords MSCs can produce more cell growth factors with a lower immunogenic potential and better immunomodulatory functions. Studies have demonstrated that MSCs extracted from the amniotic membrane and fat are superior to those collected from bone marrow, and MSCs sourced from umbilical cord blood show the weakest immunosuppressive potential
[28][29][29,30].
Adipose tissue-derived MSCs (AD-MSCs) demonstrated worse cell morphology and matrix formation than BM-MSCs during in vitro chondrogenesis, whereas their adipogenic differentiation capacity was found to be comparable
[30][31]. Although it is difficult to discriminate between the chondrogenic differentiation of BM-MSCs and AD-MSCs in monolayers, only the chondrogenic capacity of BM-MSCs was shown to be enhanced in three-dimensional cell culture
[31][32]. When compared with the same donor’s subcutaneous adipose tissue, AD-MSCs in the subpatellar fat of an osteoarthritis knee manifested an enhancement in chondrogenesis and osteogenesis
[32][33]. However, cell yields from inhaled tissues vary between BM-MSCs and AD-MSCs, with the latter having a higher survival rate than the former
[30][31]. Only 0.001–0.01% of the 6 × 10
6 nucleated cells that may be generated from a milliliter of bone marrow extract are MSCs
[33][34]. On the other hand, adipose tissue (2 × 10
6 cells per gram) contains roughly 10% bone marrow MSCs
[34][35]. Due to the absence of clinical research comparing the performance of MSCs derived from diverse sources, it is unclear which cells perform the best for OA healing. Intriguingly, OA was found to be alleviated by implanting a tiny adipose tissue depot obtained from wild-type mice or mouse embryonic fibroblasts that had spontaneously become adipocytes
[35][36].
The initially investigated MSCs were BM-MSCs; however, due to their high invasiveness and limited quantity in vivo, they have been increasingly supplanted by MSCs from other sources, the most striking of which are human umbilical-cord-derived MSCs (HUC-MSCs). Thanks to their robust in vitro proliferation capacity, minimal immunogenicity, ease of isolation and culture, and sustained multidirectional differentiation potential, HUC-MSCs are frequently utilized. HUC-MSCs suppress an inflammatory response caused by IL-1 and repair-impaired cartilage by differentiating into cartilage
[36][37]. Moreover, human-induced pluripotent stem-cell-derived MSCs (iPSC-MSCs) generated from human-induced pluripotent stem cells are a unique form of stem cells with an enhanced regenerative capacity compared with conventional stem cells, and they are able to alter highly differentiated adult somatic cells through genetic engineering. Theoretically, all adult somatic cells could be reprogrammed into iPSC-MSCs, which exhibit a higher proliferation capability than conventional MSCs. The formation of new hyaline cartilage at the joint surface defect areas following iPSC-MSC transplantation into a New Zealand rabbit model suggested the reparative action of iPSC-MSCs
[37][38]. Moreover, Cheng et al., discovered that iPSC-MSCs may release certain substances via the paracrine route to prevent the cleavage of caspase and contribute to the control of inflammation, indicating a possible function for iPSC-MSCs in immunosuppression
[38][39].
3. Underlying Treating Mechanism of MSCs for OA
The therapeutic potential of MSCs in terms of immunological and inflammatory disorders has been explored in several clinical studies
[39][40][40,41]. BM-MSCs produce substances that are immunoregulatory and anti-inflammatory
[41][42]. Therefore, MSCs are able to efficiently downregulate immune inflammatory processes and boost tissue regeneration because they display particular immunological traits and activities. When tissues are damaged, local tissue progenitor cells that raise the potential to modulate the immune system are called upon and activated
[42][43]. OA is characterized by an influx of immune cells, mostly monocytes/macrophages and then T cells, into the synovium. Furthermore, OA synovia contain mast cells, natural killer (NK) cells, dendritic cells, B cells, and granulocytes. This subject has been more thoroughly covered elsewhere
[43][44]. There might be three ways to accommodate the immune system. Firstly, BM-MSCs may control innate immunity by suppressing the development of mature dendritic cells
[44][45] and lowering the cytotoxicity of NK cells
[45][46]. Secondly, MSCs may modify acquired immunity by preventing cell death (apoptosis) and slowing the development of T and B cells
[41][46][42,47]. Finally, MSCs may switch macrophages from an inflammatory (M1) phenotype to a restorative (M2) phenotype
[47][48]. It has been demonstrated that BM-MSCs could induce a switch in macrophage phenotype from the pro-inflammatory M1 phenotype, which generates IL-1 and TGF-β, to the anti-inflammatory and chondrogenic phenotype, which produces IL-10, IL-rheumatoid arthritis, and TGF-β
[48][49]. The impact of BM-MSCs on macrophage polarization is mediated by TNF-α, which stimulates gene/protein 6 (TSG-6), prostaglandin E2 (PGE2), and indoleamine 2-dioxygenase 3-dioxygenase (IDO)
[48][49]. The immune switch from M1 to M2 could be beneficial in relieving OA by reducing periarticular inflammation. Moreover, BM-MSCs suppress pathogenic immune responses, remove infections, and improve local cell function
[49][50].
However, the precise mechanism through which BM-MSCs promote joint repair is not yet known. It would be wonderful if MSCs could be implanted and immediately develop into chondrocytes
[50][51][52][51,52,53]. Nonetheless, the survival rate of implanted MSCs was found to be low
[53][54], and 50 days after injection, BM-MSCs could not be detected
[54][55].
It has been shown in some studies that implanted MSCs may boost stem/progenitor cell recruitment and cartilage differentiation by secreting substances that encourage the proliferation and anabolism of articular chondrocytes
[53][55][56][54,56,57]. Factors produced by MSCs have been shown to affect synovium and articular chondrocytes, which control anabolic and catabolic processes
[57][58][58,59] and increase the synthesis of molecular mediators of inflammation and chondrogenesis
[53][59][60][54,60,61]. Moreover, by downregulating the neuralgia pathway, reduced inflammation may alleviate neuropathic pain.