Arsenic contamination in soil and water is a major problem worldwide. Inorganic arsenic is widely present as arsenate and arsenite. Arsenic is transferred to crops through the soil and irrigation water. It is reported to reduce crop production in plants and can cause a wide array of diseases in humans, including different types of cancers, premature delivery, stillbirth, and spontaneous abortion. Arsenic methyltransferase (AS3MT) in the human body converts inorganic arsenic into monomethylarsonic acid and dimethylarsinic acid, which are later excreted from the body. Arsenic transfer from the soil to grains of rice involves different transporters such as Lsi1, Lsi2, and Lsi6. These transporters are also required for the transfer of silicate, which makes them important for the plant.
1. Effect of Arsenic on Plants
Arsenic, when present in the soil or irrigation water, is toxic to plants
[1][22]. Although the degree of toxicity varies with the plant species, the effects are almost the same. Arsenic exhibits both physiological and morphological effects on plants. Increased lipid peroxidation, superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR) activity are common symptoms shown by plants such as soybean, rice, black gram, spinach, and barley under arsenic stress (
Table 1). Exposure to arsenic further activates phosphate transporters, which are responsible for As and phosphate uptake
[2][23].
Table 1.
Effect of arsenic on morphological and physiological parameters of different plants.
Exposure to As can bring about epigenetic changes in plants. For example, in
Pteris cretica (L.) var.
Albo-lineata, exposure to 100 mg As kg
−1 reduced the methylation at cytosine (5 mC)
[15][32]. Arsenic affects oxidative phosphorylation and adenosine triphosphate synthesis because of its similar structure to adenosine triphosphate
[16][33]. Ethylene production, membrane damage, hindered root hair growth, reduced chlorophyll content, transpiration efficiency, abscission in plant leaves, and increased oxidative stress weaken the protein synthesis system in the presence of arsenic, which in turn hinders plant growth
[7][17][18][19][14,24,34,35]. Plant exposure to As can also lead to chlorosis and necrosis
[20][36]. Arsenic accumulation in the root and shoot is dose-dependent
[21][37]. The exposure of rice to arsenate leads to reduced glutathione content, a reduced glutathione/glutathione disulfide ratio, and increased phytochelatins (PCs)
[18][34].
Ceratophyllum demersum pigments showed the first sign of arsenic toxicity, while the change in the arsenic form and its distribution was a lethal sign of toxicity. Arsenic is accumulated in young mature leaves when they are exposed to 1µM of arsenic, where it may replace phosphate or interfere with nucleic acid synthesis. The effect of As on chlorophyll is attributed to hampered synthesis rather than degradation
[22][38]. Electron transfer inhibition during photosynthesis is responsible for oxidative stress in plants under As stress
[23][39].
2. Arsenic Accumulation Depends on Plant Genotype
Different genotypes respond differently to As toxicity in terms of their biomass and length. For instance, black gram with a longer root length and more shoot weight are less affected by arsenic toxicity
[6][17]. A negative correlation of silica with arsenic was observed in temperate
japonica and tropical
japonica, but no correlation was found in
indica,
aus, and aromatic rice varieties
[24][43]. Applying silica to reduce the arsenic in the grain does not solve the problem, as silica only reduces 20% of grain As
[25][60]. The arsenic concentration in the shoots is significantly affected by genotype.
Indica genotypes accumulate less arsenite in the shoot as compared to the hybrid genotypes Xiangfengyou9 (‘XFY-9’) and Shenyou9586 (‘SY-9586’)
[26][71]. Arsenic accumulates differently in diverse wheat varieties
[4][9]. Similarly, an As-sensitive barley variety, ZDB475, accumulated about nine times the arsenic compared to the As-resistant variety ZDB160
[8][25]. The genotype also affects the shoot silicon in rice varieties, but the correlation between shoot As and shoot silicon is subpopulation specific. Temperate
japonica shows a strong correlation between shoot silicon and grain As, while the correlation is weaker in tropical
japonica, and no correlation was observed in
aus and
indica [24][43].
Bacteria can tolerate arsenic by either detoxifying it or utilizing it as an energy source
[27][72]. Bacteria use the arsenic resistance (ars) system and arsenic methylation and related pathways for detoxifying organoarsenicals and inorganic arsenic, while the arsenite oxidation system and arsenate reduction system are used for energy generation
[28][4]. When arsenic is accumulated or adsorbed by bacteria, its appearance can become wrinkly
[19][35]. Bacteria can affect the As toxicity to plants by either reducing the bioavailability, and, hence, the uptake or increasing the bioavailability and, hence, the phytoremediation
[16][33].
Halomonas sp.
Exo1 isolated from the rhizosphere of the
Avicennia marina can bioadsorb arsenic up to 43 mg kg
−1 (dry weight) of dead cell biomass
[29][73]. The secretion of exopolysaccharides may also increase the tolerance towards heavy metals, as reported for
Halomonas sp.
Exo1 against [As(III)], Cr, Cd, and Mn.
Halomonas sp.
Exo1 was reported to bioremediate As by bio adsorption on exopolysaccharide and by converting arsenite into arsenate. Halophilic bacteria AB402 and AB403 have a reported resistance against both As and Cu, but their tolerance is reduced in the presence of arsenic alone
[16][33]. The tolerance in these cells was due to the adsorption of As on extracellular substances and intracellular storage.
3. Application of Nanotechnology to Counter Negative Effects of Arsenic on Plants
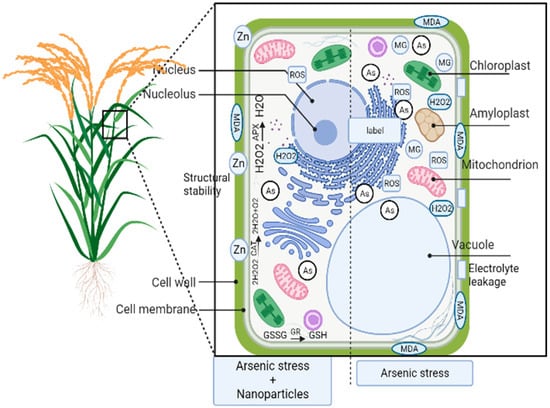
Supplementation with zinc oxide nanoparticles (ZnO–NPs) enhanced the shoot length, transpiration rate, shoot dry weight, ascorbate–glutathione cycle enzymes, net photosynthesis rate, total chlorophyll, stomatal conductance, carotenoid content, photochemical quenching, leaf relative water content, and root length under As stress compared to the control and reduced the MDA content, Methyl Glyoxal (MG) content, and electrolyte leakage in soybean plants (
Figure 13)
[30][6]. ZnO helped in reducing the MG content by enhancing the activities of Gly I and Gly II. ZnO supplies Zn to plants, which, under stress conditions, binds to sulfhydryl groups and phospholipids, maintaining their stability. Additional Zn also helps in the uptake of K, Mg, Ca, Fe, and P, hence maintaining organelle functionality
[30][6]. Wu et al.
[31][74] reported similar findings that ZnO–NPs increased the biomass, nutrients of Zn, and germination, and decreased the As uptake in rice. Iron (III) oxide nanoparticles (Fe
2O
3-NPs) have been reported to reduce the negative effects caused by As in
Vigna radiata. Ferric chelate reductase (FCR) embedded in the plasma membrane converts Fe
3+ to Fe
2+, which is then transported to the plant via the Fe(II) transporter protein. Fe
2O
3-NPs increase the root length, dry biomass, and Fe level while decreasing the root FCR activity, H
2O
2, and proline content in the seedlings, as well as the MDA content. Fe
2O
3-NPs adhere to the seedling/plant surface and adsorb As and reduce its uptake by the plant
[9][26]. Furthermore, composite NZVI@SiO2@ celluloses (FSC) showed a high adsorbent capacity, and they can be utilized for the removal of arsenic from water
[32][75]. However, their utilization for different crops needs to be tested. Magnesium oxide nanoparticles (MgO-NPs) improve the plant growth, biomass, and chlorophyll content, reduce the amount of ROS, and increase antioxidant enzyme activity
[33][76]. MgO-NPs reduce arsenic accumulation and translocation factors in a dose-dependent manner, which makes them more effective in making rice safer for consumption.
B. subtilis S4 application with iron oxide nanoparticles (IONPs) on
Cucurbita moschata enhances the plant growth under normal and arsenic stress
[34][77]. Although
B. subtilis S4 alone can enhance the chlorophyll, indole acetic acid, putrescine, spermidine content, net photosynthetic rate, CAT, SOD, and APX, it showed a synergistic effect in combination with IONPs. A similar synergistic effect can be seen in the reduction of the H
2O
2 and arsenic uptake under As stress, both of which are reported to alleviate stress.
Figure 13. B. subtilis S4 application with iron oxide nanoparticles (IONPs), magnesium oxide nanoparticles (MgO-NPs), iron (III) oxide nanoparticles (Fe
2O
3-NPs), and zinc oxide nanoparticles (ZnO–NPs) reduce arsenic uptake in plant under arsenic stress.
B. subtilis S4 with iron oxide nanoparticles (IONPs) further exerts its effect through the increased activity of catalase (CAT), glutathione reductase (GR), and ascorbate peroxidase (APX). ZnO–NPs help plants in overcoming the negative effects of arsenic stress by increasing phospholipid stability and decreasing Methyl Glyoxal (MG) content and electrolyte leakage. (Adapted from
[10][30][33][6,27,76]).
Plant Extracts Mitigate Arsenic Effects
The As uptake by rice was reduced by 70% by the addition of Neem (
Azadirachta indica) or tulsi (
Ocimum sanctum) extract
[34][77]. Both extracts also restored plant biomass and length, and decreased H
2O
2 and OH
-, thereby protecting the seedlings by lowering lipid peroxidation. Most importantly, both extracts seem to protect protein thiols and protein carbonylation under arsenic toxicity. β-pinene at a concentration of 10µM reduces the effect of As on the root and shoot length, along with reducing the As accumulation and H
2O
2 content, but has no significant effect on lipoxygenase (LOX) and MDA
[23][39]. β-pinene can protect plants by providing stability to the membrane and inducing a systemic acquired resistance. It can quench singlet oxygen species due to the presence of a double bond and provide membrane stability under Cr and As stress
[23][35][39,78]. Volatile oil and aqueous extracts of
Rosmarinus officinalis can reduce the chromosome aberrations caused by arsenic exposure in Allium cepa and promote DNA repair
[36][79].