You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Vidushi Neergheen-Bhujun and Version 3 by Jessie Wu.
Cancer is responsible for lifelong disability and decreased quality of life. Cancer cells undergo numerous changes in their metabolic pathways, involving energy and biosynthetic processes, so that they can proliferate. Hence, the metabolic pathways appear as interesting targets for a broad spectrum of therapeutic approaches. Mushrooms possess biological activities relevant to disease-fighting and to the prevention of cancer. They have a long-standing tradition of use in ethnomedicine and have been included as an adjunct therapy during and after oncological care. Mushroom-derived compounds have also been reported to target the key signature of cancer cells in in vitro and in vivo studies.
- cancer
- conjugated linoleic acid
- GL22
- grifolin
- metabolic reprogramming
- neoalbaconol
1. Neoalbaconol Induced Energy Depletion and Multiple Types of Cell Death by Targeting Phosphoinositide-Dependent Kinase 1-Phosphoinositide 3-Kinase/Protein Kinase BK-1-PI3K/AKT Signalling Pathway
Albatrellus confluens is a member of the Albatrellaceae family and is mainly distributed in North America, Europe, and eastern Asia [1][2][52,62]. Several biologically active secondary metabolites with anticancer potential have been isolated from this polypore mushroom [3][4][5][63,64,65]. Recently, neoalbaconol (NA), a small-molecule with a drimane-type sesquiterpenoid structure, was extracted from the fruiting body of this fungus [6][66] (Figure 1).
Figure 1.
Structures of mushroom-derived compounds that target energy-relevant regulators in cancer.
Deng et al. [6][66] demonstrated that NA can significantly inhibit the proliferation of nasopharyngeal carcinoma cell lines (C666-1, HK1, SUNE1, HNE2-LMP1, CNE1-LMP1, and 5-8F), breast cancer cell lines (ZR75-1, MX-1, T47D, MAD-MB-231, MDA-MB-453, and MCF-7), colon cancer cell lines (HCT116 and SW620), leukaemia cell line (K562), prostate cancer cell line (DU145), lung adenocarcinoma epithelial cell line (A549), and melanoma cell line (A375) in a time- and dose-dependent manner. The latter did not affect the proliferation of immortalised normal cell lines (human keratinocyte HaCaT, human nasopharynx epithelial NP69, and mouse fibroblast NIH/3T3) at high doses. This underscored the selectivity of the constituent isolated from A. confluens toward cancer cells with C666-1, HK1, and ZR75-1 cells reported to being more sensitive to NA. Flow cytometry, protein, and confocal and electron microscope assays have shown that apoptosis and necroptosis were responsible for the death-inducing efficacy of NA and the cleavage of poly (ADP-ribose) polymerase-1 by caspases, and the interaction and colocalisation of endogenous receptor-interacting protein 1 and receptor-interacting protein 3 were observed in C666-1 cells. This is because by docking into the adenosine triphosphate (ATP)-binding pocket of phosphoinositide-dependent kinase 1 (PDK1) and inhibiting its kinase activity, NA inhibited the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway and its downstream metabolic regulator HK2 and autophagy, which was activated by c-JUN N-terminal kinases to provide a survival advantage, was unable to reverse the NA-induced energy depletion stress and cell death.
Furthermore, Deng et al. [6][66] also evaluated the in vivo efficacy of the secondary metabolite (100 mg/kg/day) in C666-1 induced tumours in athymic nude mice. The average tumour volume was 2.4 times smaller in the NA-treated group compared with the vehicle-treated group. None of the mice showed signs of toxicity and the average tumour weight of the NA-treated group and control group was 0.65 ± 0.23 g and 1.26 ± 0.32 g, respectively, at the treatment end point. Consistent with the in vitro data, the in vivo data outlined the efficacy of NA in suppressing tumour progression by inhibiting the AKT signalling pathway (Figure 2).
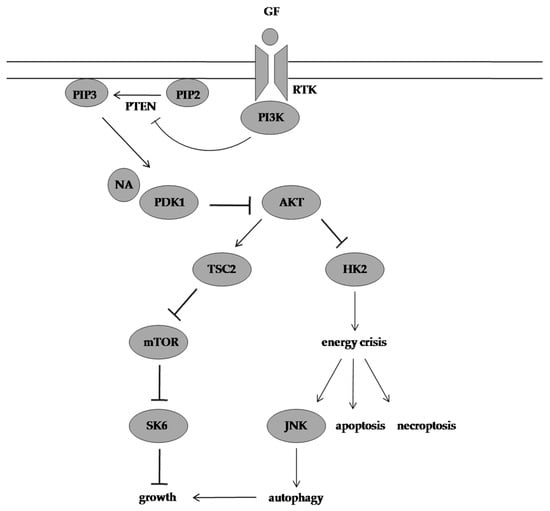
Figure 2. Molecular mechanism of action of NA. NA targets PDK1 and suppresses its downstream effectors resulting in energy crisis, and autophagy provides a survival advantage in NA-treated cells but is unable to prevent cell death in response to this energy depletion. GF: growth factor, RTK: receptor tyrosine kinase, PI3K: phosphoinositide 3-kinase, PIP2: phosphatidylinositol-4,5-biphosphate, PTEN: phosphatase and tensin homolog, PIP3: phosphatidylinositol-3,4,5-triphosphate, NA: neoalbaconol, PDK1: phosphoinositide-dependent kinase 1, AKT: protein kinase B, TSC2: tuberous sclerosis 2, mTOR: mammalian target of rapamycin, SK6: p70 S6 kinase, HK2: hexokinase 2, JNK: c-JUN N-terminal kinase.
2. GL22 Suppressed Tumour Growth by Altering Lipid Homeostasis and Triggering Cell Death
Ganoderma is a cosmopolitan genus of polypore fungi in the family Ganodermataceae and is commonly known as Ling Chu, Ling Zhi, reishi, and the mushroom of immortality [1][7][8][9][10][51,52,67,68,69]. These species contain polysaccharides, fatty acids, steroids, triterpenoids, and alkaloids as bioactive constituents and have been traditionally administered throughout Asia for centuries as a cancer treatment and are highly sought-after and of great economic value [11][70].
Wang et al. [12][71] isolated GL22, a triterpene farnesyl hydroquinone hybrid, from the fruiting body of G. leucocontextum, and Liu et al. [13][72] evaluated the bioactivity of this compound (Figure 1). They reported that GL22 displayed growth-inhibitory activity against Huh7.5 cells with a half-maximal inhibitory concentration (IC50) value of 8.9 μM and haematoxylin and eosin staining has revealed that a Huh7.5 xenograft tumour treated with GL22 displayed enlarged intercellular spaces and decreased cell density relative to the control. This natural compound exerted inhibitive effects in cell line experiments and animal modelling by altering cellular lipid homeostasis. In fact, GL22 treatment significantly decreased the expression levels of peroxisome proliferator-activated receptor alpha and peroxisome proliferator-activated receptor gamma, which subsequently led to the downregulation of the mRNA levels of fatty acid-binding protein 1, fatty acid-binding protein 4, and fatty acid-binding protein 5. These intracellular lipid chaperones reversibly bind fatty acids and coordinate their import, transport, storage, and export, and by antagonising the transcriptional levels of fatty acid binding proteins, GL22 blocked fatty acid transport. This GL22-mediated immobilisation of free fatty acids led to a sharp increase in the average number and size of lipid droplets, and this observation was consistent with impaired cardiolipin biosynthesis. This signature phospholipid of the mitochondria is involved in mitochondrial biogenesis, mitochondrial bioenergetics, and mitochondrial dynamics, and the GL22-induced abnormality in cardiolipin content altered the mitochondrial shape and size and caused membrane integrity damage and fragmentation of the mitochondrial cristae. This eventually triggered mitochondrial dysfunction, reduced ATP production, and decreased aerobic respiration in Huh7.5 cells.
Furthermore, GL22 treatment induced apoptosis in liver cancer cells by upregulating the levels of p53 and Bcl-2-associated X protein and downregulating the level of Bcl-2. Similarly, the hallmarks of the mitochondrial-mediated intrinsic apoptotic pathways, which include caspase 3, caspase 9, and poly (ADP-ribose) polymerase cleavage, and the hallmarks of the death receptor-mediated extrinsic apoptotic pathways, which include caspase 8 cleavage, were activated by GL22 treatment (Figure 3).
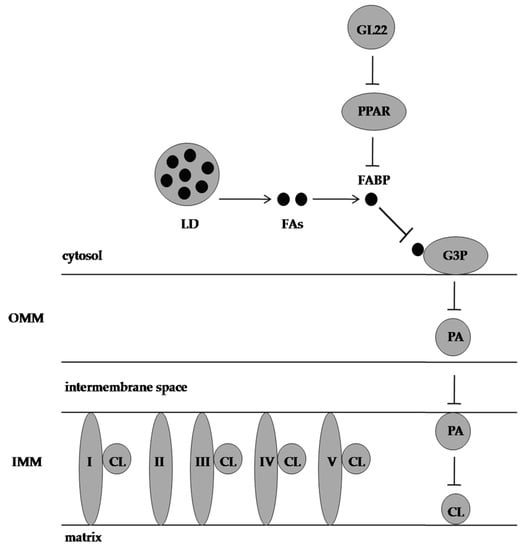
Figure 3. Effect of GL22 on Huh7.5 cells. CL is needed for oxidative phosphorylation complexes assembly and is synthesised from G3P and FAs. GL22 displays antimetabolic activity by downregulating FABP expression and inhibiting CL biosynthesis. PPAR: peroxisome proliferator-activated receptor, LD: lipid droplet, FAs: fatty acids, FABP: fatty-acid-binding protein, G3P: glycerol-3-phosphate, PA: phosphatidic acid, CL: cardiolipin, OMM: outer mitochondrial membrane, IMM: inner mitochondrial membrane, I, II, III, IV, and V represent electron transport chain complexes.
3. Grifolin Reversed DNA Methyltransferase T1-Mediated Metabolic Reprogramming Induced by Epstein-Barr Virus Latent Membrane Protein 1
Epstein-Barr virus (EBV) is responsible for lymphoid and epithelial malignancies, and this oncovirus infection is characterised by the expression of latent genes, which include Epstein-Barr nuclear antigens, Epstein-Barr nuclear antigen leader protein, latent membrane protein 1 (LMP1), latent membrane protein 2, non-coding EBV-encoded RNAs, and viral microRNA [14][73]. Among them, LMP1 is a driver oncogene in nasopharyngeal carcinoma and plays an imperative role in its pathogenesis by upregulating HK2, activating the fibroblast growth factor 2/fibroblast growth factor receptor 1 signalling pathway, and increasing the expression of glucose transporter 1 through the mammalian target of rapamycin complex 1/nuclear factor kappa B signalling pathway [15][16][17][74,75,76].
Luo et al. [18][77] have documented that aerobic fermentation in CNE1-LMP1 cells was markedly enhanced by 90% compared to LMP1-negative CNE1 cells, and this is primarily because LMP1 downregulated the phosphatase and tensin homolog/AKT (PTEN/AKT) signalling pathway in a DNA methyltransferase 1 (DNMT1)-dependent manner. Furthermore, LMP1 also switched glucose metabolism from oxidative phosphorylation to aerobic glycolysis in nasopharyngeal carcinoma cells by promoting DNMT1 mitochondrial translocation, which further led to an increase in the methylation/unmethylation (M/U) ratio of DNA fragments in the mitochondrial DNA D-loop region and a decrease in the DNA levels of MT-COXII, MT-ATP6, and MT-ND6 that encode cytochrome c oxidase subunit II, ATP synthase F0 subunit 6, and NADH dehydrogenase 6, respectively. These observations were consistent with the metabolic flux measurements, which showed that the 13C-labelled lactate level was significantly increased and the 13C-labelled tricarboxylic acid (TCA) cycle metabolite levels, which included 13C-α-ketoglutarate, 13C-citrate, 13C-fumarate, and 13C-malate, were significantly decreased in CNE1-LMP1 cells compared with CNE1 cells.
Grifolin, a farnesyl phenolic compound extracted from the mushrooms A. confluens and Boletus pseudocalopus, which previously suppressed the growth and metastasis of HeLa, MCF-7, SW480, K562, and MG63 tumour cell lines, was reported to decrease the amount of glucose used for lactate production in LMP1-positive cells by targeting DNMT1 to demethylate and reactivate the PTEN gene (Figure 1). Furthermore, grifolin treatment significantly attenuated DNMT1 retention in the mitochondria of CNE1-LMP1 cells, and the M/U ratio of the mitochondrial DNA D-loop region was consequently decreased in these cells. Similarly, grifolin promoted subunit assembly to form oxidative phosphorylation complexes, and it also ensured that more nicotinamide adenine dinucleotide is used by complex I to enhance respiration in CNE1-LMP1 cells (Figure 4).
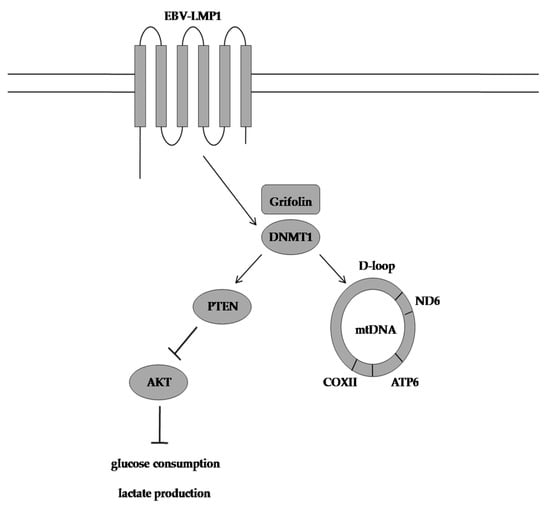
Figure 4. Grifolin-induced mode of action. Grifolin restores oxidative phosphorylation in nasopharyngeal carcinoma cells by reactivating the PTEN gene and reducing the M/U ratio of the mitochondrial DNA D-loop region. EBV-LMP1: Epstein-Barr virus latent membrane protein 1, DNMT1: DNA methyltransferase 1, PTEN: phosphatase and tensin homolog, AKT: protein kinase B, COXII: cytochrome oxidase subunit II, ATP6: ATP synthase membrane subunit 6, ND6: NADH ubiquinone oxidoreductase core subunit 6, M/U ratio: methylation and unmethylation ratio.
Luo et al. [18][77] also reported that grifolin can phenocopy the effect of DNMT1 inhibitor 5-AZA-2-deoxycytidine and, unlike this epidrug, grifolin is chemically stable and not accompanied by serious side effects and, thus, can be used as a safe alternative to improve tumour control.
4. Conjugated Linoleic Acid Exhibited Proapoptotic Effects by Counteracting Altered Lipid Metabolism
Agaricus is the type genus of the family Agaricaceae in the phylum Basidiomycota and is distributed worldwide [19][20][21][78,79,80]. These saprobic mushrooms are often gregarious in forests, pasture land, grass land, rubbish dumps, manure heaps, and alluvial soil and include economically important species, such as A. bisporus, which is also known as white button mushroom, table mushroom, cultivated mushroom, portobello mushroom, and crimini mushroom [19][22][23][24][25][78,81,82,83,84]. This genus of macrofungi is widely used and extensively studied for its dietetic, ethnopharmacological, and medicinal properties, and the literature abounds on the topic [26][27][28][29][30][31][85,86,87,88,89,90].
Chen et al. [32][91] have identified conjugated linoleic acid (CLA) as the main constituent of an ethyl acetate extract of A. bisporus, and they reported that CLA decreased breast cancer cell proliferation by inhibiting aromatase activity (Figure 1). Adams et al. [33][92] investigated the anticancer potential of CLA in androgen-sensitive LNCaP and androgen-insensitive PC3 and DU145 prostate cancer cell lines. The mushroom extract decreased cell proliferation in all cell lines tested in a dose-dependent manner compared with untreated control cells, and the magnitude of response was similar between the cell lines. The Cell Death Detection ElisaPlus Photometric Enzyme Immunoassay and the Annexin V Assay results highlighted that the mushroom extract induced DNA fragmentation and phosphatidylserine translocation in the prostate cancer cell lines, but LNCaP cells were more prone to the proapoptotic effects of CLA, and this underscored that the antiproliferative activity is different from the proapoptotic activity of the extract because the cell lines under study responded equally in the Real-Time Proliferation Assay. Furthermore, Adams et al. [33][92] also evaluated the prostate cancer protective effects of A. bisporus in vivo by using male athymic mice injected with either PC3 or DU145 prostate cancer cells, and the LNCaP cell line was not used for animal experiments because it did not form tumours when implanted. The in vivo studies have shown that the oral intake of the mushroom extract decreased PC3 tumour weight and DU145 tumour weight by 68.6% and 44.5%, respectively, compared with the pair-fed control mice. Histological examinations of PC3 and DU145 tumours have revealed that cell proliferation decreased by 45% and 25.3%, respectively, in the mushroom-extract-fed group compared with the control, and the level of apoptosis was significantly increased in DU145 tumours.
The in vitro results were in line with the in vivo results, and the microarray analysis suggested several mechanisms for the CLA effect on prostate cancer cell proliferation and apoptosis. The CLA-rich mushroom extract upregulated the expression of FAS/APO-1 gene which plays a central role in the physiological regulation of apoptosis by 2.84-fold and downregulated the expression of KIT gene, which is involved in the proliferation and survival of cells by four-fold. Similarly, the extract inhibited diacylglycerol production and eventually increased apoptosis through increased production of arachidonic acid and ceramide and also decreased cyclooxygenase-2 protein expression and subsequently suppressed the conversion of arachidonic acid to prostaglandin E2, which fosters cancer progression by (1) blocking apoptosis through the activation of the PI3K/AKT/peroxisome proliferator-activated receptor signalling pathway, (2) increasing angiogenesis through vascular endothelial growth factor activation, (3) inducing immune suppression by increasing interleukin 10 production, and (4) increasing cancer cell proliferation through the activation of rat sarcoma virus/rapidly accelerated fibrosarcoma/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signalling pathway. The aforesaid effects on lipid metabolism were accompanied by the inhibition of isocitrate dehydrogenase 2 (IDH2) and the increased expression of fumarate hydratase (FH). The three-fold decrease in IDH2 expression normalised the TCA cycle in prostate cancer cells, and the 4.3-fold increase in FH expression inhibited fumarate build-up in prostate cancer cells and deactivated the angiogenic factor hypoxia-inducible factor 1-alpha (Figure 5).

Figure 5. CLA-inducing apoptosis in prostate cancer cell lines by inhibiting DAG production, increasing AA and ceramide production, and inhibiting IDH2 activity. PLC: phospholipase C, CLA: conjugated linoleic acid, DAG: diacylglycerol, PKC: protein kinase C, AA: arachidonic acid, COX-2: cyclooxygenase-2, 5-LOX: 5-lipooxygenase, PGE2: prostaglandin E2, SMase: sphingomyelinase, α-KG: alpha-ketoglutarate, IDH2: isocitrate dehydrogenase.
Adams et al. [33][92] also observed that the ethyl acetate extract downregulated the expression of the endothelin 1 gene, which is commonly upregulated in hypoxia by three-fold. Furthermore, the extract also upregulated the expression of immune-related genes, such as those encoding interleukin 15, S100 calcium binding protein A8, S100 calcium binding protein A9, and lectin, galactosidase-binding soluble, 1 proteins.
