Naturally occurring hydroxycinnamic acids, namely ferulic, para-coumaric, caffeic, sinapic acids, and 3,4-dimethoxycinnamic acid are present in significant quantities in the cell wall of plants. They can make up about a third of the phenolic compounds in our diet, hence the interest in their potential biological activity and properties. Methods of synthesis and properties of natural and synthetic hydroxycinnamic acid derivatives are reviewed in detail.
- hydroxycinnamic acids
- natural compounds
- antioxidant
- hydroxicinnamic acids synthesis
1. Introduction
Hydroxycinnamic acids (HCAs) are a class of phenolic compounds whose characteristic structural feature is the phenolic ring and a radical containing a carboxyl group (Figure 1). These compounds differ from each other by substituents on the phenolic ring. Naturally occurring HCAs, namely ferulic, para-coumaric, caffeic, sinapic acids, and 3,4-dimethoxycinnamic acid (3,4-DMCA) are present in significant quantities in the cell wall of plants. In plants, these compounds are formed through the mevalonate-shikimate biosynthesis pathway, which allows plants to synthesize essential amino acids from the primary metabolites phosphoenolpyruvate and erythrose-4-phosphate [27][1]. Upon deamination of phenylalanine and tyrosine, trans-cinnamic and coumaric acids, respectively, are synthesized. The routes of synthesis and further conversions of HCAs are shown in Figure 1. Some of them are of particular interest since they display various confirmed biological activities and are widespread in plants.
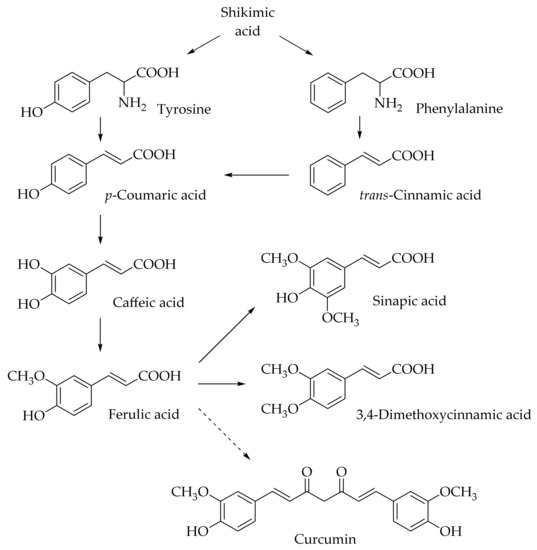
Figure 1. Biosynthesis and structures of naturally occurring hydroxycinnamic acids and curcumin.
2. Foods of Plant Origin
HCAs are ubiquitous in foods of plant origin and can make up about a third of the phenolic compounds in our diet [28][2]. The predominant HCA in most fruits is caffeic acid, accounting for more than 75% of the total amount of HCA derivatives found in plums, apples, apricots, blueberries, and tomatoes [29][3]. However, p-coumaric acid is predominant in citrus fruits and pineapples [30][4]. Generally, HCAs are present in the bound form and are rarely found in a free form. Processing of the fruits and vegetables (freezing, sterilization, and fermentation) promotes the formation of free HCAs in plant products. The most common water-soluble derivatives of HCAs are esters between the carboxyl group of the phenolic acid and one of the alcohol groups of the organic compound, for example, chlorogenic acids (esters of HCAs and quinic acid), and glucosides with a glycosidic bond with the hydroxyl group of the HCA derivative (for example, p-coumaric acid O-glucoside).
Ferulic acid is one of the most abundant phenolic compounds in the cell wall of plants. Ferulic acid can be found in all living species, from bacteria to humans. It can be absorbed in the small intestine and excreted in the urine. The content of ferulic acid in plants is considerable, varying from 5 g/kg in a wheat bran to 9 g/kg in the pulp of a sugar beet and 50 g/kg in the kernel of a corn [31][5]. It is found mainly in seeds and leaves, both in free form and covalently linked to lignin and other biopolymers. It is usually found in the form of esters, cross-linked with polysaccharides in the cell wall, such as arabinoxylans in herbs, pectin in spinach and sugar beets, and xyloglucans in bamboo. Crosslinking with proteins is also possible. Ferulic acid is usually found in the highest concentration in grains and has also been found in plant foods such as burdock, eggplant, grapefruits, oranges, soybean, and bamboo shoots [32][6]. Caffeic acid was found but not quantified in significant amounts in foods such as cereals and cereal products, arabica coffee, fruits, plants, oils, grapes, and tea [33][7]. Sinapic acid is found in dietary plants such as rye, citrus and berry fruits, vegetables, cereals, and oilseed crops [34–36][8][9][10].
The daily intake of caffeic and ferulic acids in humans is estimated to be 500–1000 mg from fruits, vegetables, grains, bran, beer, and coffee [37][11]. HCAs in a free form are absorbed by monocarboxylic acid transporters in the mucous membrane of the gastrointestinal tract. The absorption efficiency largely depends on the affinity of the compounds for monocarboxylic acid transporters, and when HCAs were administered to rats, it was shown that the affinity scale grows in the order of caffeic acid < p-coumaric acid = ferulic acid [38][12]. The peak plasma content for ferulic acid is reached 5–10 min after oral administration [38,39][12][13].
3,4-DMCA and its derivatives, which are components of black coffee, and especially green coffee beans, are noteworthy [40–44][14][15][16][17][18]. These compounds are bioavailable and active in the body through the use of coffee [45,46][19][20].
In fact, the plant Coffea canephora var. robusta contains a higher level of 3,4-DMCA (X ±SD: 0.433 ± 0.15, in the range of 0.237 to 0.691 g/kg) than Coffea arabica (X ±SD: 0.059 ± 0.03, in the range from 0.016 to 0.095 g/kg) [47][21].
3,4-DMCA is of particular interest because it appears in large quantities (~380 nM for 60 min) in human blood plasma after coffee uptake, despite the low concentration in the coffee extract. Ferulic acid also appears in plasma in a free form after coffee consumption. Interestingly, 3,4-DMCA constitutes only 3% of the ferulic acid content in coffee extract, but its concentration reaches 2.4-times higher than that of the ferulic acid in blood plasma after drinking coffee [41][15]. The 3,4-DMCA in coffee extract is found as a conjugate with quinic acid, but after ingestion of coffee, all 3,4-DMCA is found in free-form in plasma samples [41][15]. It has been shown that hydrolysis of 3,4-DMCA derivatives by intestinal esterases occurs in the small intestine [48][22].
Finally, it is worth mentioning curcumin (diferuloylmethane), which is not formally a HCA derivative, but has common structural features with them (Figure 1). Curcumin is a natural dye and is derived from the roots of the Curcuma longa plant. A previous study has shown the ability of curcumin to overcome the blood-brain barrier, which indicates its potential to be used for the prevention and treatment of various neurodegenerative diseases [49][23].
3. Synthesis and Properties of Natural and Synthetic Hydroxycinnamic Acid Derivatives
There are three major routes for the preparation of the fundamental cinnamic acid scaffold: carboxyl group formation, synthesis of a propenoic acid fragment or addition of an aromatic radical on the propenoic acid moiety [50][24]. The most common and straightforward methods for the synthesis of cinnamic acid itself is via the Perkin reaction[25] [51] or Knoevenagel condensation[26] [52] starting from benzaldehyde (Figure 2). Even though the latter procedure can also be used for the synthesis of HCA derivatives, many other methods for constructing a cinnamoyl moiety have been also developed (Figure 2), e.g., reaction of benzaldehydes with Meldrum’s acid in the presence of catalytic amounts of 2-carbamoylhydrazine-1-sulfonic acid and carbamoylsulfamic acid [53][27], Wittig olefination reaction of benzaldehydes [54[28][29],55], Heck coupling of aryl halides or benzoic anhydride with alkenes[30] [56], and straightforward synthesis from aromatic aldehydes, aliphatic carboxylic acids and boron tribromide using 4-dimethylaminopyridine and pyridine as bases [57][31]. Here, we will focus only on synthetic methods for the preparation of HCAs and their derivatives.
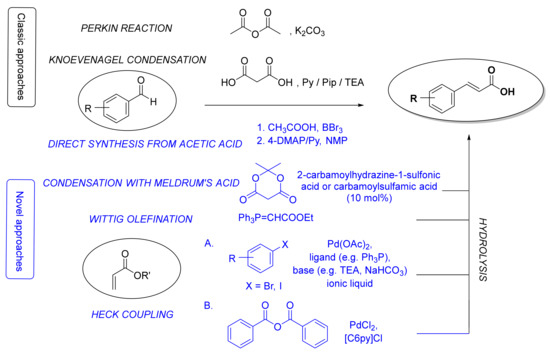
Figure 2. The most common classic (in black) and novel (in blue) synthetic methods for the synthesis of cinnamic acid moieties. Py, pyridine; Pip, piperidine; TEA, triethylamine; 4-DMAP, 4-dimethylaminopyridine; NMP, N-methyl-2-pyrrolidone; [C6Py]Cl, N-hexylpyridinium chloride.
In the past ten years, there has been a trend to replace the classic synthetic methodologies with novel and preferably eco-friendly/green procedures involving mild reaction conditions, minimal waste and low energy consumption. One successful eco-sustainable example is visible light photoredox catalysis (Figure 3), which implements visible light as a clean and cheap energy source, and CBr4 in alkaline aqueous solution providing the carboxylic acid moiety. (E)-4-(2-nitrovinyl)phenol and CBr4 as coupling partners were thus used for a highly stereoselective synthesis of (E)-p-coumaric acid in high 83% yield [58][32]. New green chemistry approaches have commonly employed water as the main solvent. Thiemann and coworkers described the Wittig reaction between 4-hydroxybenzaldehyde and ethoxymethylidenetriphenylphosphorane in 10% aqueous sodium hydroxide (Figure 3), which enabled one-pot olefination-hydrolysis sequence reactions leading to p-coumaric acid in situ in 74% yield [55][29]. The main methoxycinnamic acids were also synthesized, e.g., 3,4-dimethoxycinnamic acid (3,4-DCMA) was obtained in excellent 96% yield. Similarly, alkaline aqueous solution was used for the Heck coupling reaction between 4-iodophenol and acrylic acid, in which p-coumaric acid was prepared in excellent 95% yield in the presence of potassium hydroxide [59][33]. A green chemistry approach employing edible fruits and vegetable, liqueurs, and waste waters (buttermilk and residues of olive processing) as solvents with Meldrum’s acid and appropriate aldehyde under ultrasound irradiation provided p-coumaric, caffeic and ferulic acids in excellent yields [60][34]. Another modern strategy was to perform the reactions under solvent-free conditions. One such example was developed by van Schijndel and coauthors using environmentally benign amines or ammonium salts which afforded α,β-unsaturated acids from corresponding benzaldehydes and malonic acid via Knoevenagel condensation. Sinapic, ferulic and p-coumaric acid were prepared in moderate to high yields from 3,5-dimethoxy-4-hydroxy-, 3-methoxy-4-hydroxy- and 4-hydroxy-benzaldehydes, respectively; however, with higher temperatures (i.e., 140 °C), second decarboxylation usually occurred [61][35]. Recently, van Schijndel and coauthors published a similar procedure at 90 °C that led to aforementioned HCAs in excellent yields (Figure 3) [62][36]. Furthermore, to limit the decarboxylation of the resulting HCAs, another approach employing microwave irradiation was presented in the literature. Microwave-assisted Knoevenagel–Doebner condensation of various para-hydroxybenzaldehydes with malonic acid and piperidine as a base in dimethylformamide (Figure 3) afforded p-coumaric, caffeic, ferulic and sinapic acids in 92, 85, 89, and 90% yield, respectively [63][37]. Furthermore, the addition of tetrabutylammonium bromide (TBAB) in Knoevenagel reaction afforded quick, economic and eco-friendly microwave-assisted synthesis of p-coumaric acid from 4-hydroxybenzaldehyde and malonic acid in 72% yield using potassium carbonate as a base and distilled water as a solvent [64][38]. p-Coumaric, 4-methoxycinnamic and 3,4-dimethoxycinnamic acids were also synthesized in 80, 85 and 92% yield in solvent-free conditions and microwave irradiation using polyphosphate ester as a reaction mediator and catalyst [65][39]. Recent studies on HCA synthesis have also focused on the development of novel catalysts for Knoevenagel–Doebner condensation or the Heck reaction (Figure 3), e.g., nanochannels of hexagonal mesoporous carbon nitride with optimized pore sizes [66[40][41],67], amphiphilic and hyperbranched polymer-functionalized magnetic nanoparticles with palladium [68][42], and bismuth(III) chloride [69][43]. Using these catalysts, all major HCAs and methoxycinnamic acids were synthesized (Figure 3). The reaction of aryl iodides in NaOH (aq) with copper as a catalyst and l-(−)-quebrachitol as a ligand (Figure 3) afforded ferulic, isoferulic and 3- and 4-coumaric acids in high yields [70][44]. Another interesting approach was combining microwave and ultrasound irradiation to accelerate the Knoevenagel–Doebner reaction in aqueous media [71][45]. The optimized green method provides the rapid synthesis of HCAs with low energy consumption, minimal waste and without any organic solvents and expensive catalysts. One recent synthetic methodology also employed a recyclable silicon-containing biphenyl-based template affording the Pd-catalyzed para C-H functionalization of numerous phenolic compounds as starting materials (e.g. phenol and 2-methoxyphenol were converted to p-coumaric acid and ferulic acid in both 4 steps and 47% yields, respectively) [72][46]. And last but not least, HCAs and derivatives can also be prepared via enzymatic reactions [73–75][47][48][49], most commonly performed by microbes, e.g., bacteria.
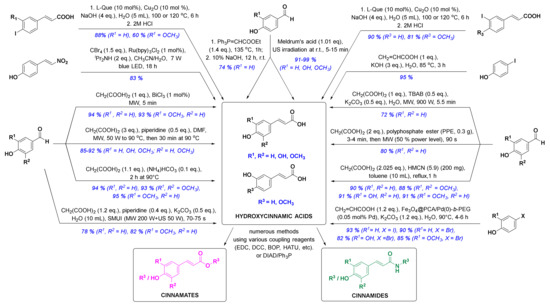
Figure 3. Novel synthetic methods for the synthesis of HCA derivatives (reagents are presented in black, yields and substituents in blue). l-Que, l-(−)-quebrachitol; LED, light emitting diode; MW, microwave irradiation; DMF, dimethylformamide; SMUI, simultaneous microwaves-ultrasound irradiation; US, ultrasound; TBAB, tetrabutylammonium bromide; PPE, polyphosphate ester; HMCN, hexagonal mesoporous carbon nitride; Fe3O4@PCA/Pd(0)-b-PEG, hyperbranched poly(ethylene glycol)-block-poly(citric acid)-functionalized Fe3O4 magnetic palladium nanoparticles; EDC, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide; DCC, dicyclohexylcarbodiimide; BOP, benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; HATU, 1-(bis(dimethylamino)methylene)-1H-[1,2,3]triazolo[4 ,5-b]pyridine-1-ium 3-oxide hexafluorophosphate; DIAD, diisopropyl azodicarboxylate.
Natural HCAs possess numerous biological activities that have been extensively reviewed in several publications [76–84][50][51][52][53][54][55][56][57][58]. In the last 20 years, the chemistry-driven derivatization of HCAs has led to a large number of semi-synthetic and synthetic HCA derivatives. In order to obtain specific biological effects, HCAs were most commonly converted to corresponding esters or amides. For example, an ester of p-coumaric acid (methyl (E)-(3-(4-hydroxyphenyl)acryloyl)-l-phenylalaninate) and two esters of caffeic acid (dibenzyl (E)-(3-(3,4-dihydroxyphenyl)acryloyl)-l-aspartate and methyl (E)-(3-(3,4-dihydroxyphenyl)acryloyl)-l-alaninat) were synthesized by condensation of the corresponding cinnamic acids with amino acid esters using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), 1-hydroxybenzotriazole (HOBT), and triethylamine in methylene chloride [85]. The first two HCA esters exhibited anti-atherosclerotic activity via inhibitory effects on acyl-CoA:cholesterol acyltransferase involved in cellular cholesterol storage and transport, and inhibition of LDL-oxidation and high-density lipoprotein particle size rearrangement [85][59]. The same coupling method (for the synthesis of amides) or alternative esterification utilizing diisopropyl azodicarboxylate and triphenylphosphine was used for preparation of a series of caffeic, ferulic and p-coumaric acids amides with biogenic amines (serotonin, dopamine, tyramine, vanillylamine) or esters with 3,4-dihydroxyphenethyl, 4-hydroxyphenethyl, and phenethyl alcohols [86][60]. Compounds containing catechol, o-methoxyphenol or 5-hydroxyindole moieties exhibited potent 1,1-diphenyl-2-picrylhydrazyl free radical scavenging activity (amides more potent than esters). Some HCA derivatives also showed potent and selective MAO-B (phenethyl (E)-3-(4-hydroxyphenyl)acrylate being the most potent one) or moderate BChE (e.g., 3,4-dihydroxyphenethyl (E)-3-(4-hydroxyphenyl)acrylate) inhibitory activity [86][60]. The synthesis of phenethyl E-3-(4-hydroxy-3-methoxyphenyl)acrylate and E-3-(4-hydroxy-3-methoxyphenyl)-N-phenethylacrylamide from ferulic acid with phenethyl alcohol and phenethylamine, respectively, was performed via indirect reaction, including acetylation for hydroxyl group protection, acid chloride formation, esterification/amidation, and deacetylation as a deprotection reaction [87][61]. Both compounds showed anticancer activity against P388 murine leukemia cells. Other coupling reagents, i.e., benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate(BOP), 1-(bis(dimethylamino)methylene)-1H-[1,2,3]triazolo[4,5-b]pyridine-1-ium 3-oxide hexafluorophosphate (HATU), and dicyclohexylcarbodiimide (DCC) have been used for the preparation of ferulic[62] [88] and caffeic acid esters and amides [89][63]. The nucleophilic hydroxyl groups were often protected via acetylation by acetic anhydride in the presence of pyridine [87,90,91][61][64][65] or other bases [92,93][66][67]. Other methods for the preparation of HCA esters and amides have been used and were thoroughly reviewed by de Armas-Ricard and co-authors in 2019 [89][63].
As previously described, esters and amides of coumaric, ferulic and caffeic acid possess numerous biological activities, e.g., antioxidant [82[56][57][60][63][68][69][70],83,86,89,94–96], anticancer [78[52][61][66],87,92], antibacterial [82[56][71][72][73],97–99], antifungal [100–102][74][75][76], antiviral[56][64][77] [82,90,103], anti-inflammatory [104–106][78][79][80], and many other activities [84,107,108][58][81][82]. The most commonly described health beneficial properties of HCAs and their derivatives is antioxidant activity due to the phenolic hydroxyl group(s) on the main aromatic ring, which possess(es) the ability to react with free radicals and reactive oxygen species forming a resonance-stabilized phenoxyl radical. Furthermore, propenoic side chain involvement in the stabilization of a phenoxyl radical via a conjugated double bond also contributes to antioxidant properties of HCAs [82,109][56][83]. Their antioxidant potential is affected by substituents on the phenyl ring (e.g., methoxy group on position 3 increases the antioxidant potency due to electron-donating properties).
The substitution on the aromatic ring has also a high impact on their physico-chemical properties. Among the main cinnamic acid derivatives, higher solubility was observed for caffeic acid, followed by ferulic and trans-cinnamic acids due to the extent of hydrogen bonding with water—a higher number of hydroxyl groups leads to increased association interactions with water [110][84]. The hydroxy substituent also shows a significant impact on the acidity of HCAs [111][85]. For coumaric acids, the pKa values for carboxylic acid group (pKa1) increases in the following order: orto < meta < para (4.11, 4.49 and 4.70, respectively), whereas for phenolic group (pKa2) the m-coumaric acid is the least acidic (pKa2 = 10.39) [111][85]. On the other hand, the apparent acid dissociation constants (pKaapp) of trans-cinnamic, ferulic and caffeic acids were determined as 4.55, 4.61 and 4.77, respectively [110][84].
References
- K. M. Herrmann; The Shikimate Pathway as an Entry to Aromatic Secondary Metabolism. Plant Physiology 1995, 107, 7-12, 10.1104/pp.107.1.7.
- José Teixeira; Alexandra Gaspar; E. Manuela Garrido; Jorge M.P.J. Garrido; Fernanda Borges; Hydroxycinnamic Acid Antioxidants: An Electrochemical Overview. BioMed Research International 2013, 2013, 1-11, 10.1155/2013/251754.
- C Manach; Augustin Scalbert; Christine Morand; Christian Rémésy; Liliana Jiménez; Polyphenols: food sources and bioavailability. The American Journal of Clinical Nutrition 2004, 79, 727-747, 10.1093/ajcn/79.5.727.
- Macheix, J.; Fleuriet, A.; Billot, J. The main phenolics of fruits. In Fruit Phenolics; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 1990; pp. 1–98.
- Shiyi Ou; Kin-Chor Kwok; Ferulic acid: pharmaceutical functions, preparation and applications in foods. Journal of the Science of Food and Agriculture 2004, 84, 1261-1269, 10.1002/jsfa.1873.
- Zhaohui Zhao; Mohammed H. Moghadasian; Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chemistry 2008, 109, 691-702, 10.1016/j.foodchem.2008.02.039.
- K.H Janbaz; S.A Saeed; Anwarul-Hassan Gilani; Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine 2004, 11, 424-430, 10.1016/j.phymed.2003.05.002.
- Sawa, T.; Nakao, M.; Akaike, T.; Ono, K.; Maeda, H. Alkylperoxyl radical-scavenging activity of various flavonoids and other phenolic compounds: Implications for the anti-tumor-promoter effect of vegetables. J. Agric. Food Chem. 1999, 47, 397–402.
- Koski, A.; Pekkarinen, S.; Hopia, A.; Wähälä, K.; Heinonen, M. Processing of rapeseed oil: Effects on sinapic acid derivative content and oxidative stability. Eur. Food Res. Technol. 2003, 217, 110–114.
- Moreno, D.A.; Pérez-Balibrea, S.; Ferreres, F.; Gil-Izquierdo, Á.; García-Viguera, C. Acylated anthocyanins in broccoli sprouts. Food Chem. 2010, 123, 358–363.
- Michael N Clifford; Chlorogenic acids and other cinnamates – nature, occurrence, dietary burden, absorption and metabolism. Journal of the Science of Food and Agriculture 2000, 80, 1033-1043, 10.1002/(sici)1097-0010(20000515)80:7<1033::aid-jsfa595>3.3.co;2-k.
- Konishi, Y.; Zhao, Z.; Shimizu, M. Phenolic acids are absorbed from the rat stomach with different absorption rates. J. Agric. Food Chem. 2006, 54, 7539–7543.
- Yang, C.; Tian, Y.; Zhang, Z.; Xu, F.; Chen, Y. High-performance liquid chromatography–electrospray ionization mass spectrometry determination of sodium ferulate in human plasma. J. Pharm. Biomed. Anal. 2007, 43, 945–950.
- Nagy, K.; Redeuil, K.; Williamson, G.; Rezzi, S.; Dionisi, F.; Longet, K.; Destaillats, F.; Renouf, M. First identification of dimethoxycinnamic acids in human plasma after coffee intake by liquid chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 491–497.
- Farrell, T.L.; Gomez-Juaristi, M.; Poquet, L.; Redeuil, K.; Nagy, K.; Renouf, M.; Williamson, G. Absorption of dimethoxycinnamic acid derivatives in vitro and pharmacokinetic profile in human plasma following coffee consumption. Mol. Nutr. Food Res. 2012, 56, 1413–1423.
- Grosso, G.; Stepaniak, U.; Topor-Mądry, R.; Szafraniec, K.; Pająk, A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 2014, 30, 1398–1403.
- Adisakwattana, S. Cinnamic Acid and Its Derivatives: Mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients 2017, 9, 163.
- Kiyama, R. Estrogenic Activity of Coffee Constituents. Nutrients 2019, 11, 1401.
- Monteiro, M.; Farah, A.; Perrone, D.; Trugo, L.C.; Donangelo, C. Chlorogenic Acid Compounds from Coffee Are Differentially Absorbed and Metabolized in Humans. J. Nutr. 2007, 137, 2196–2201.
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic Acids from Green Coffee Extract are Highly Bioavailable in Humans. J. Nutr. 2008, 138, 2309–2315.
- Paula B. Andrade; Raquel Beatrize Leitão; R.M Seabra; M. Beatriz Oliveira; M.A Ferreira; 3,4-Dimethoxycinnamic acid levels as a tool for differentiation of Coffea canephora var. robusta and Coffea arabica. Food Chemistry 1998, 61, 511-514, 10.1016/s0308-8146(97)00067-8.
- Mette F. Andreasen; Paul A. Kroon; Gary Williamson; Maria-Teresa Garcia-Conesa; Esterase Activity Able To Hydrolyze Dietary Antioxidant Hydroxycinnamates Is Distributed along the Intestine of Mammals. Journal of Agricultural and Food Chemistry 2001, 49, 5679-5684, 10.1021/jf010668c.
- Shri K. Mishra; Kalpana Palanivelu; The effect of curcumin (turmeric) onAlzheimer′s disease: An overview. Annals of Indian Academy of Neurology 2008, 11, 13-19, 10.4103/0972-2327.40220.
- A. V. Simonyan; Activity of cinnamic acid derivatives and new methods for their synthesis (review). Pharmaceutical Chemistry Journal 1993, 27, 92-100, 10.1007/bf00781068.
- Wang, Z. Perkin Reaction. In Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 2160–2163.
- Kolb, K.E.; Field, K.W.; Schatz, P.F. A One-Step Synthesis of Cinnamic Acids Using Malonic Acid: The Verley-Doebner Modification of the Knoevenagel Condensation. J. Chem. Educ. 1990, 67, A304.
- Mohammad Ali Zolfigol; Roya Ayazi-Nasrabadi; Saeed Baghery; Synthesis and characterization of two novel biological-based nano organo solid acids with urea moiety and their catalytic applications in the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol), coumarin-3-carboxylic acid and cinnamic acid derivatives under mild and green conditions. RSC Adv. 2015, 5, 71942-71954, 10.1039/c5ra14001c.
- Speed, T.J.; McIntyre, J.P.; Thamattoor, D.M. Wittig Reaction Using a Stabilized Phosphorus Ylid: An Efficient and Stereoselective Synthesis of Ethyl trans-Cinnamate. J. Chem. Educ. 2004, 81, 1355.
- Thiemann, T.; Elshorbagy, M.W.; Salem, M.H.F.A.; Ahmadani, S.A.N.; Al-Jasem, Y.; Al Azani, M.; Al-Sulaibi, M.A.M.; Al-Hindawi, B. Facile, Direct Reaction of Benzaldehydes to 3-Arylprop-2-Enoic Acids and 3-Arylprop-2-Ynoic Acids in Aqueous Medium. Int. J. Org. Chem. 2016, 06, 126–141.
- Adrian J. Carmichael; Martyn J. Earle; John David Holbrey; Paul B. McCormac; Kenneth R. Seddon; The Heck Reaction in Ionic Liquids: A Multiphasic Catalyst System. Organic Letters 1999, 1, 997-1000, 10.1021/ol9907771.
- Constantin I. Chiriac; Fulga Tanasa; Marioara Onciu; A Novel Approach in Cinnamic Acid Synthesis: Direct Synthesis of Cinnamic Acids from Aromatic Aldehydes and Aliphatic Carboxylic Acids in the Presence of Boron Tribromide. Molecules 2005, 10, 481-487, 10.3390/10020481.
- Shubhangi Tripathi; Lal Dhar S. Yadav; Visible-light-enabled denitrative carboxylation of β-nitrostyrenes: a direct photocatalytic approach to cinnamic acids. New Journal of Chemistry 2018, 42, 3765-3769, 10.1039/c7nj04578f.
- Amit Shard; Naina Sharma; Richa Bharti; Sumit Dadhwal; Rajesh Kumar; Arun K. Sinha; Tandem Heck/Decarboxylation/Heck Strategy: Protecting?Group?Free Synthesis of Symmetric and Unsymmetric Hydroxylated Stilbenoids†. Angewandte Chemie 2012, 124, 12416-12419, 10.1002/ange.201206346.
- Serena Fiorito; Vito Alessandro Taddeo; Salvatore Genovese; Francesco Epifano; A green chemical synthesis of coumarin-3-carboxylic and cinnamic acids using crop-derived products and waste waters as solvents. Tetrahedron Letters 2016, 57, 4795-4798, 10.1016/j.tetlet.2016.09.023.
- Jack Van Schijndel; Luiz Alberto Canalle; Dennis Molendijk; J Jan Meuldijk; The green Knoevenagel condensation: solvent-free condensation of benzaldehydes. Green Chemistry Letters and Reviews 2017, 10, 404-411, 10.1080/17518253.2017.1391881.
- Jack Van Schijndel; Dennis Molendijk; Koen Van Beurden; Luiz Alberto Canalle; Timothy Noël; Jan Meuldijk; Preparation of bio-based styrene alternatives and their free radical polymerization. European Polymer Journal 2020, 125, 109534, 10.1016/j.eurpolymj.2020.109534.
- Louis M. M. Mouterde; Florent Allais; Microwave-Assisted Knoevenagel-Doebner Reaction: An Efficient Method for Naturally Occurring Phenolic Acids Synthesis. Frontiers in Chemistry 2018, 6, 426, 10.3389/fchem.2018.00426.
- Monika Gupta; Basant Purnima Wakhloo; Tetrabutylammoniumbromide mediated Knoevenagel condensation in water: synthesis of cinnamic acids. Arkivoc 2007, 2007, 94, 10.3998/ark.5550190.0008.110.
- A. Mobinikhaledi; N. Foroughifar; H. Fathinejad Jirandehi; Microwave–Assisted Synthesis of Cinnamic Acid Derivatives in the Presence of PPE and under Solvent-Free Condition. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry 2008, 38, 428-430, 10.1080/15533170802254602.
- Elamathi, P.; Chandrasekar, G.; Muthuraman, S.; Kolli, M.K. Pore size engineering of hexagonal mesoporous carbon nitride (HMCN) for high catalytic performance in the synthesis of α, β-unsaturated acid and its derivatives. Appl. Surf. Sci. 2019, 463, 481–491.
- Elamathi, P.; Chandrasekar, G. Pore Size Architecture of Hexagonal Mesoporous Carbon Nitride (HMCN) for Metal-Free Synthesis of p-Hydroxycinnamic Acid. Catal. Letters 2018, 148, 1758–1767.
- Seyed Jamal Tabatabaei Rezaei; Azin Shamseddin; Ali Ramazani; Asemeh Mashhadi Malekzadeh; Pegah Azimzadeh Asiabi; Palladium nanoparticles immobilized on amphiphilic and hyperbranched polymer-functionalized magnetic nanoparticles: An efficient semi-heterogeneous catalyst for Heck reaction. Applied Organometallic Chemistry 2017, 31, e3707, 10.1002/aoc.3707.
- None Suresh; Dhruva Kumar; Jagir S. Sandhu; Bismuth(III) Chloride–Mediated, Efficient, Solvent-Free, MWI-Enhanced Doebner Condensation for the Synthesis of (E)-Cinnamic Acids. Synthetic Communications 2010, 40, 1915-1919, 10.1080/00397910903162833.
- Xinjie Liang; Hui Li; Fangyu Du; Yongsheng Zhang; Jinhua Dong; Xuefei Bao; Ying Wu; Guo-Liang Chen; Copper and l-(−)-quebrachitol catalyzed hydroxylation and amination of aryl halides under air. Tetrahedron Letters 2020, 61, 152222, 10.1016/j.tetlet.2020.152222.
- Yanqing Peng; Gonghua Song; Combined microwave and ultrasound accelerated Knoevenagel–Doebner reaction in aqueous media: a green route to 3-aryl acrylic acids. Green Chemistry 2003, 5, 704-706, 10.1039/b310388a.
- Tuhin Patra; Sukdev Bag; Rajesh Kancherla; Anirban Mondal; Aniruddha Dey; Sandeep Pimparkar; Soumitra Agasti; Atanu Modak; Debabrata Maiti; Palladium-Catalyzed DirectedparaC−H Functionalization of Phenols. Angewandte Chemie International Edition 2016, 55, 7751-7755, 10.1002/anie.201601999.
- Hong, J.; Im, D.-K.; Oh, M.-K. Investigating E. coli Coculture for Resveratrol Production with 13 C Metabolic Flux Analysis. J. Agric. Food Chem. 2020, 68, 3466–3473.
- Katsoura, M.H.; Polydera, A.C.; Tsironis, L.D.; Petraki, M.P.; Rajačić, S.K.; Tselepis, A.D.; Stamatis, H. Efficient enzymatic preparation of hydroxycinnamates in ionic liquids enhances their antioxidant effect on lipoproteins oxidative modification. New Biotechnol. 2009, 26, 83–91.
- Vargas-Tah, A.; Gosset, G. Production of Cinnamic and p-Hydroxycinnamic Acids in Engineered Microbes. Front. Bioeng. Biotechnol. 2015, 3.
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293.
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499.
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic Acid Derivatives as Anticancer Agents-A Review. Curr. Med. Chem. 2011, 18, 1672–1703.
- El-Seedi, H.R.; Taher, E.A.; Sheikh, B.Y.; Anjum, S.; Saeed, A.; AlAjmi, M.F.; Moustafa, M.S.; Al-Mousawi, S.M.; Farag, M.A.; Hegazy, M.-E.F.; et al. Hydroxycinnamic Acids: Natural Sources, Biosynthesis, Possible Biological Activities, and Roles in Islamic Medicine. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 269–292.
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, Natural Sources, Dietary Intake, Pharmacokinetic Properties, and Biological Activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 60, 10877–10895.
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370.
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767.
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190.
- Taofiq, O.; González-Paramás, A.; Barreiro, M.; Ferreira, I. Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a Review. Molecules 2017, 22, 281.
- Sangku Lee; Jong-Min Han; Hyunjung Kim; Eungsoo Kim; Tae-Sook Jeong; Woo Song Lee; Kyung-Hyun Cho; Synthesis of cinnamic acid derivatives and their inhibitory effects on LDL-oxidation, acyl-CoA:cholesterol acyltransferase-1 and -2 activity, and decrease of HDL-particle size. Bioorganic & Medicinal Chemistry Letters 2004, 14, 4677-4681, 10.1016/j.bmcl.2004.06.101.
- Koichi Takao; Kazuhiro Toda; Takayuki Saito; Yoshiaki Sugita; Synthesis of Amide and Ester Derivatives of Cinnamic Acid and Its Analogs: Evaluation of Their Free Radical Scavenging and Monoamine Oxidase and Cholinesterase Inhibitory Activities. Chemical and Pharmaceutical Bulletin 2017, 65, 1020-1027, 10.1248/cpb.c17-00416.
- Firdaus; N H Soekamto; Seniwati; M F Islam; Sultan; Phenethyl ester and amide of Ferulic Acids: Synthesis and bioactivity against P388 Leukemia Murine Cells. Journal of Physics: Conference Series 2018, 979, 012016, 10.1088/1742-6596/979/1/012016.
- Ayyoub Selka; Fanta J. Ndongou Moutombi; Marc Cormier; Mohamed Touaibia; Phenethyl Esters and Amide of Ferulic Acid, Hydroferulic Acid, Homovanillic Acid, and Vanillic Acid: Synthesis, Free Radicals Scavenging Activity, and Molecular Modeling as Potential Cholinesterases Inhibitors. Molbank 2020, 2020, M1151, 10.3390/M1151.
- Merly De Armas-Ricard; Enrique Ruiz-Reyes; Oney Ramírez-Rodríguez; Caffeates and Caffeamides: Synthetic Methodologies and Their Antioxidant Properties. International Journal of Medicinal Chemistry 2019, 2019, 1-15, 10.1155/2019/2592609.
- Wang, P.; Liu, C.; Sanches, T.; Zhong, Y.; Liu, B.; Xiong, J.; Neamati, N.; Zhao, G. Design and synthesis of novel nitrogen-containing polyhydroxylated aromatics as HIV-1 integrase inhibitors from caffeic acid phenethyl ester. Bioorg. Med. Chem. Lett. 2009, 19, 4574–4578.
- Yang, C.; Zhao, J.; Cheng, Y.; Le, X.C.; Rong, J. N -Propargyl Caffeate Amide (PACA) Potentiates Nerve Growth Factor (NGF)-Induced Neurite Outgrowth and Attenuates 6-Hydroxydopamine (6-OHDA)-Induced Toxicity by Activating the Nrf2/HO-1 Pathway. ACS Chem. Neurosci. 2015, 6, 1560–1569.
- Cai, H.; Huang, X.; Xu, S.; Shen, H.; Zhang, P.; Huang, Y.; Jiang, J.; Sun, Y.; Jiang, B.; Wu, X.; et al. Discovery of novel hybrids of diaryl-1,2,4-triazoles and caffeic acid as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase for cancer therapy. Eur. J. Med. Chem. 2016, 108, 89–103.
- Touaibia, M.; Guay, M. Natural Product Total Synthesis in the Organic Laboratory: Total Synthesis of Caffeic Acid Phenethyl Ester (CAPE), A Potent 5-Lipoxygenase Inhibitor from Honeybee Hives. J. Chem. Educ. 2011, 88, 473–475.
- Menezes, J.C.J.M.D.S.; Kamat, S.P.; Cavaleiro, J.A.S.; Gaspar, A.; Garrido, J.; Borges, F. Synthesis and antioxidant activity of long chain alkyl hydroxycinnamates. Eur. J. Med. Chem. 2011, 46, 773–777.
- Shahidi, F.; Chandrasekara, A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem. Rev. 2010, 9, 147–170.
- Szwajgier, D.; Pielecki, J.; Targoński, Z. Antioxidant activities of cinnamic and benzoic acid derivatives. Acta Sci. Pol. Technol. Aliment. 2005, 4, 129–142.
- López, M.A.; Rodríguez, Z.; González, M.; Tolón, B.; Avila, R.; González, I.; Garmendía, L.; Mamposo, T.; Carrasco, R.; Pellón, R.; et al. Novel cephalosporin derivatives possessing a substituted cinnamoyl moiety at the 7β-position. Synthesis, structural characterization and antibacterial activity of 3-acetoxymethyl cephalosporin derivatives. Eur. J. Med. Chem. 2004, 39, 657–664.
- Narasimhan, B.; Belsare, D.; Pharande, D.; Mourya, V.; Dhake, A. Esters, amides and substituted derivatives of cinnamic acid: Synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2004, 39, 827–834.
- Yingyongnarongkul, B.; Apiratikul, N.; Aroonrerk, N.; Suksamrarn, A. Solid-phase synthesis and antibacterial activity of hydroxycinnamic acid amides and analogues against methicillin-resistant Staphylococcus aureus and vancomycin-resistant S. aureus. Bioorg. Med. Chem. Lett. 2006, 16, 5870–5873.
- Korošec, B.; Sova, M.; Turk, S.; Kraševec, N.; Novak, M.; Lah, L.; Stojan, J.; Podobnik, B.; Berne, S.; Zupanec, N.; et al. Antifungal activity of cinnamic acid derivatives involves inhibition of benzoate 4-hydroxylase (CYP53). J. Appl. Microbiol. 2014, 116, 955–966.
- Tawata, S.; Taira, S.; Kobamoto, N.; Zhu, J.; Ishihara, M.; Toyama, S. Synthesis and Antifungal Activity of Cinnamic Acid Esters. Biosci. Biotechnol. Biochem. 1996, 60, 909–910.
- Zhou, K.; Chen, D.; Li, B.; Zhang, B.; Miao, F.; Zhou, L. Bioactivity and structure-activity relationship of cinnamic acid esters and their derivatives as potential antifungal agents for plant protection. PLoS ONE 2017, 12, e0176189.
- Seung Uk Lee; Cha-Gyun Shin; Chong-Kyo Lee; Yong Sup Lee; Caffeoylglycolic and caffeoylamino acid derivatives, halfmers of l-chicoric acid, as new HIV-1 integrase inhibitors. European Journal of Medicinal Chemistry 2007, 42, 1309-1315, 10.1016/j.ejmech.2007.02.016.
- Boudreau, L.H.; Picot, N.; Doiron, J.; Villebonnet, B.; Surette, M.E.; Robichaud, G.A.; Touaibia, M. Caffeoyl and cinnamoyl clusters with anti-inflammatory and anti-cancer effects. Synthesis and structure–activity relationship. New J. Chem. 2009, 33, 1932.
- Kim, E.O.; Min, K.J.; Kwon, T.K.; Um, B.H.; Moreau, R.A.; Choi, S.W. Anti-inflammatory activity of hydroxycinnamic acid derivatives isolated from corn bran in lipopolysaccharide-stimulated Raw 264.7 macrophages. Food Chem. Toxicol. 2012, 50, 1309–1316.
- Nagasaka, R.; Chotimarkorn, C.; Shafiqul, I.M.; Hori, M.; Ozaki, H.; Ushio, H. Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem. Biophys. Res. Commun. 2007, 358, 615–619.
- Neelam; Khatkar, A.; Sharma, K.K. Phenylpropanoids and its derivatives: Biological activities and its role in food, pharmaceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2020, 60, 2655–2675.
- De Oliveira Silva, E.; Batista, R. Ferulic Acid and Naturally Occurring Compounds Bearing a Feruloyl Moiety: A Review on Their Structures, Occurrence, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 580–616.
- F Natella; M Nardini; M Di Felice; C Scaccini; Benzoic and cinnamic acid derivatives as antioxidants: structure-activity relation.. Journal of Agricultural and Food Chemistry 1999, 47, 1453–1459.
- Fátima L. Mota; António J. Queimada; Simão P. Pinho; Eugénia A. Macedo; Aqueous Solubility of Some Natural Phenolic Compounds. Industrial & Engineering Chemistry Research 2008, 47, 5182-5189, 10.1021/ie071452o.
- Ali Benvidi; Abbas Dadras; Saleheh Abbasi; Marzieh D. Tezerjani; Masoud Rezaeinasab; Reza Tabaraki; Mansour Namazian; Experimental and computational study of the p K a of coumaric acid derivatives. Journal of the Chinese Chemical Society 2019, 66, 589-593, 10.1002/jccs.201800265.
