1. Introduction
The emerging field of sensors offers a wide versatile platform for the detection of various analytes, which can be tailored based on the desired analyte, employing appropriate detection elements. Sensors comprise a sensing element that responds to the analyte and a transducer that produces the readable output. If the sensing element involves a chemical molecule, the sensor is classified as a chemical sensor, while those that employ biomolecules such as enzymes, antibodies, aptamers, oligonucleotides, etc., are classified as biosensors
[1][23]. While chemical sensors are cost-effective and stable, they do not possess the specificity and sensitivity of biosensors
[1][23]. Sensors are also classified based on the transduction element as optical, electrochemical, electrical, calorimetric, and piezoelectric sensors. Among the various transduction methods, electrochemical sensors are widely employed for analytical applications due to the simple operation, rapid response, cost-effectiveness, portability, and ease of fabrication
[2][24]. Though there are several reports of HbA1c quantification using optical
[3][25], photoacoustic
[4][26], electrochemical piezoelectric
[5][27], chemiluminescence
[6][28], photoelectric
[7][29], magnetic immunoassay
[8][30], etc., majority of the reports have focused on electrochemical methods of transduction owing to their merits. This re
svie
archw focuses only on the progress in electrochemical strategies for HbA1c measurement. The electrochemical sensors can be further classified as potentiometric sensors, that measure the charge potential accumulated in the working electrode, amperometric sensors, that measure the change in current due to reduction and oxidation of electroactive species in the system at a constant potential, voltametric sensors, that measure the current change at a defined potential window, and impedimetric sensors, that detect changes in the capacitance and charge conductance at the electrode/electrolyte interface
[9][31].
In order to improve the sensitivity, response times, and detection range of the electrochemical sensors, the earlier generations of electrochemical sensors employed an electron transfer agent or a redox mediator, while the current generation of sensors employ direct electron transfer on an electrode surface that is modified with nano-dimensional particles. These nanointerfaces offer amplified electro-catalytical, electron transfer, and conductivity owing to their structural characteristics. Nanostructures of various sizes, shapes, and chemical nature have been explored as interface materials. Further, these nanostructured interfaces also serve as immobilization matrices with a high surface area for retaining biological capture elements such as enzymes and antibodies, thereby augmenting the specificity, sensitivity, and detection range of the biosensor.
Electrochemical measurements of HbA1c have been carried out by both chemical and biosensors. In the chemical method, the conventional cis-diol interaction between boronate and the sugar moiety of HbA1c is exploited for the detection of HbA1c, while biosensors quantify the redox changes or current flow variations owing to interactions between HbA1c and the biological recognition elements, such as antibodies or enzymes. Additionally, HbA1c has also been quantified in an indirect method which involves the detection of fructosyl-valine (FV), a small peptide generated by digesting HbA1c by oxidation using the enzyme fructosyl amino acid oxidase (FAO), resulting in the generation of H
2O
2, which is measured electrochemically
[10][32]. The present re
svie
arch w includes science citation indexed articles published from the year 2000 to present in Science Direct, PubMed, and Google Scholar on electrochemical sensors for HbA1c. Sensors fabricated with either chemical or biological sensing elements have been considered for the re
searchview. Nano-interfaced and point-of-care sensors for HbA1c have been emphasized upon. Similar interfaces and electrode modification strategies have been excluded for minimizing redundancy.
2. Electrochemical Sensors for Direct HbA1c Detection Using Boronate Chemistry
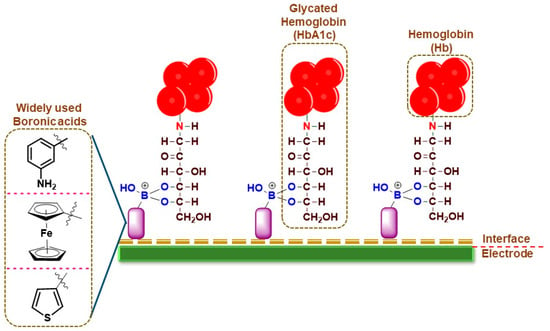
For HbA1c sensing, the electrochemical sensors work on the principle of boronic acid interactions with the diol groups in HbA1c, which are absent in normal Hb, thereby conferring specificity for the detection (Figure 1). The frequently used boronic acid derivatives are ferrocene boronic acid (FcBA), aminophenyl boronic acid (APBA), formylphenyl boronic acid (FPBA), thiophene-3-boronic acid (T3BA), etc. These boronic acid derivatives have been sometimes used in tandem with metallic nanoparticles for increased conductivity and sensing performance (Table 1).
Figure 1.
Pictorial representation of broadly used boronic acid-assisted electrochemical sensors for HbA1c detection.
In an earlier work, an amperometric sensor employing FcBA as the capture element was used for HbA1c detection. The pyrolytic graphite working electrode (PGE) modified with zirconium dioxide (ZrO
2) nanoparticles was used to immobilize Hb or HbA1c from the samples. The working electrode was then incubated in FcBA solution and the amount of HbA1c was measured through the electrochemical response of FcBA using square wave voltammetry (SWV). The electrochemical response of heme was used to quantify total hemoglobin (tHb) in the PGE without the addition of FcBA. The sensor exhibited a linear response between 6.8 and 14% of HbA1c. The sensor performance was validated using whole blood samples from human volunteers and compared with the conventional HPLC method. A variation ranging from −10.7 to 31% was observed
[11][33]. The limitations of this system are the tedious and long sample analysis time, the narrow detection range, as well as greater deviations from the values reported by the conventional methods.
Screen-printed electrodes have garnered much attention in the clinical diagnosis domain owing to their cost-effectiveness and disposability. Liu et al.
[12][34] designed a novel HbA1c sensor using a screen-printed reticulated vitreous carbon (RVC) electrode. For fabrication of the sensor, RVC foam was compressed to form a working electrode of diameter 3 mm and length 5 mm, which was further incubated in HNO
3 and H
2SO
4 solution for 1 h at 85 °C. Then, APBA was immobilized on the working electrode using an ethyl (dimethyl amino propyl) carbodiimide–N-hydroxysuccinimide (EDC-NHS) coupling reaction. Finally, the electrode was immersed in glycine solution to block the unreacted carboxylic ends of RVC. This modified RVC was set into a cylindrical polyether ether ketone (PEEK) to form the flow cell. The electrochemical properties of the electrode were studied in the flow injection assay system. The results showed that the sensor exhibited a detection range of 0.2–12.0 mg/mL HbA1c with a limit of detection (LoD) of 89 μg/mL at a low potential of +0.2 V. The electrode was also stable and lost only 4.4% of the signal intensity after 100 cycles of use.
Another disposable APBA-based sensor fabricated on screen-printed carbon electrodes (SPCE) comprised of a layer containing electrodeposited gold nanoparticles (AuNPs) that was dipped in a 1 mM TTBA (2,2′:5′,5″-terthiophene-3′-p-benzoic acid) solution. The TTBA was then electro-polymerized by potential cycling twice from 0.0 to +1.4 V (Ag/AgCl) in 0.1 M phosphate-buffered saline (PBS) (pH 7.4) at a scan rate of 0.1 V/s, followed by immobilization of APBA using EDC-NHS chemistry. The final working electrode (APBA-pTTBA/AuNPs/SPCE) when subjected to electrochemical studies demonstrated the specificity and stability of the HbA1c owing to the highly stable conducting polymer layer. The sensor had a LoD of the sensor of 0.052% and showed good correlation with conventional HPLC in the HbA1c values measured for diabetic and normal patients
[13][35]. In a similar strategy, 4-mercaptophenylboronic acid (4-MPBA) was immobilized by drop-casting on a SPCE working electrode modified with an electrodeposited layer of gold nanoflowers (AuNF). The resulting 4-MPBA/AuNF/SPCE was characterized and then employed for the quantification of HbA1c and exhibited a linear range between 5 μg/mL and 1 mg/mL of HbA1c
[14][36].
In a different approach, a boronic acid-modified gold electrode was fabricated by dipping the electrode in a DMSO (dimethyl sulfoxide) solution of 3,3′-Dithio-bis-propionic acid N-hydroxysuccinimide ester (DTSP) to form an amine-reactive self-assembled monolayer (SAM). The electrode was then incubated in a 0.5% (
w/
w) ethanolic solution of poly(amidoamine) generation 4 (PAMAM) dendrimer and then in 1 mM of 4-formyl-phenylboronic acid (FPBA). The sensing strategy employs a unique “backfilling” assay scheme, where after addition of the sample containing HbA1c, periodate-treated activated glucose oxidase (GOx) was made to react with the unreacted amine groups on the PAMAM-FPBA electrode, which produced the electrochemical response. The signal intensity decreased with increased concentrations of HbA1c due to steric interference of HbA1c on the catalytic efficiency of GOx, thereby providing a stable, quantitative measurement of HbA1c in the sample. The sensor exhibited a detection range of 2.5–15%, which was well within the clinical levels for diagnosis. However, the sensor requires further validation with clinical samples to establish its diagnostic potential
[15][37]. The same group have also reported an interface comprising a conjugate of cysteamine and FPBA that were allowed to react for 4 h at 60 °C under stirring conditions to obtain the Cys-FPBA
2 conjugate. The gold electrodes were dipped in Cys-FPBA
2 for the formation of a boronate-modified SAM on the working electrode. The electrochemical response was obtained using the backfilling method with GOx. The Hb was separated from whole blood by zinc-induced precipitation. The sensor exhibited a detection range of 4.5–15%. The sensor performance was evaluated with a small sample size of human samples. Though promising, the sensor preparation involves a larger number of steps, which increased the detection time
[16][38].
Poly(3-aminophenyl boronic acid) (PAPBA) nanoparticles obtained by polymerization of APBA have been employed as a thin-film nanointerface material over SPCE for detection of HbA1c
[17][39]. The sensor exhibited a linear range between 0.975 and 156 μM of HbA1c when measured using differential pulse voltammetry and was stable when used in the presence of interferents such as dopamine, ascorbic acid, glucose, uric acid, and bovine serum albumin. However, the sensor did not account for potential errors arising due to variations in the total hemoglobin levels.
Recently, a novel interface of anthraquinone boronic acid (AQBA) that was formed by electrochemical oxidation of 2-anthraceneboronic acid (ANBA) on pre-anodized SPCE, designated as AQBA/SPCE*, was employed for HbA1c quantification
[18][40]. The sensor performance remained unaffected in the presence of interferents such as ascorbic acid, uric acid, dopamine, glucose, fructose, mannose, and hemoglobin. The sensor detected HbA1c in the linear range of 31.2–500 nM. An AQBA-free electrode was used for detecting Hb. The sensor exhibited good correlation with the results obtained from the conventional HPLC method for HbA1c in patient samples.
Several studies have employed electrochemical impedance spectroscopic (EIS) measurements to measure the rate of charge transfer between the electrode and the redox probe, which is influenced the blocking effect caused by binding of HbA1c with the recognition element (boronic acid derivatives). An earlier study had employed screen-printed platinum electrodes, surface-coated with a fibrous mesh of eggshell membrane, incorporating APBA through glutaraldehyde crosslinking. The hemolyzed sample was immobilized on the electrode surface and changes in the impedance were used for the label-free quantification of HbA1c between 2.3 and 14%. A ferro-ferricyanide couple was employed as the redox mediator in this study
[19][41]. The study employed centrifugation to remove other glycated moieties that could potentially interfere with the analysis. While this is beneficial for the accuracy of measurement, it also makes the process time-consuming and tedious. In a similar strategy, interdigitated gold electrodes modified with a self-assembled monolayer of cysteamine covalently linked to APBA through glutaraldehyde were employed for quantification of HbA1c using impedance measurements. Hemolyzed whole blood was immobilized on the electrode surface for the measurements. The sensor exhibited a linear range of 0.1–8.36% of HbA1c with a LoD of 0.024%
[20][42]. However, the clinical utility of this system could be improved with an increase in the upper threshold of detection. Use of gold electrodes also reduces the cost-effectiveness of the measurement. In a similar study employing EIS for quantification of HbA1c, gold electrodes modified with a self-assembled monolayer of T3BA were employed to achieve a detection range between 0.08 and 8.4% of HbA1c
[21][43]. The sample preparation in this case involved removal of glycated albumin by centrifugation to avoid interferences from other glycated species in the sample. However, this additional step will not only increase the total analysis time, but also restrict the portability of the system.
Graphene oxide (GO) modified with APBA has also been employed for the impedimetric-based quantification of HbA1c. In this work, 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), a coupling agent, was employed to facilitate the formation of an amide bond between the carboxylic group of GO and the amine group of APBA. The synthesized GO-APBA was drop-casted onto a clean glassy carbon electrode and employed for further electrochemical studies. The sensor could detect HbA1c in the range of 8%
[22][44]. However, the study did not specify the limit of detection, linear range of detection of HbA1c, or the effect of interferents on the sensor performance. Therefore, the true clinical effectiveness of this system could not be predicted.
Hsieh et al. developed a ring-shaped interdigitated electrode (RSIDE) that works on impedance spectroscopy for the quantification of HbA1c. Here, the gold electrode surface was modified with a SAM of T3BA. The sensor design was evaluated on a computational platform using COSMOL simulations, which was then translated to a biosensor chip for quantifying HbA1c. The sensor measured HbA1c in the range of 1–100 mg/L. The cheap, label-free, and easy micro-electro-mechanical system (MEMS) fabrication of the sensor can be effectively translated to a point-of-care diagnostic device, provided validation with real patient samples is demonstrated
[23][45].
Recently, a SPCE with a nanointerface comprising bovine serum albumin (BSA), multi-walled carbon nanotubes (MWCNTs), and glutaraldehyde (GA) was reported for measurement of HbA1c. The BSA-MWCNT composite in PBS was crosslinked to the electrode by using glutaraldehyde, followed by immobilization of APBA using EDC-NHS coupling. The BSA imparted an anti-fouling nature to the electrode surface. The fabricated sensor detected HbA1c between 2 and 15% in the samples. To improve the sensitivity of the sensor, the working electrode was further modified by incorporation of the anti-HbA1c antibody. The sensor also evaluated unprocessed human serum
[24][46]. However, the use of antibodies on the electrode surface could lead to increased costs and a reduced shelf-life.
Table 1.
Boronic acid-based electrochemical sensors for direct detection of HbA1c.
NA: Not applicable.
3. Electrochemical Immunosensors for HbA1c Detection Using Antibodies
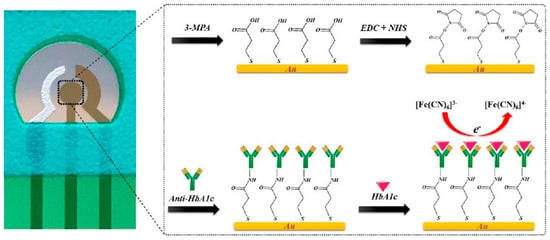
Antibodies against HbA1c have been employed as specific capture agents to selectively retain HbA1c on the electrode surface. The captured HbA1c could result in increased impedance or could be quantified using an enzyme-linked secondary antibody for generating the electrochemical response. Molazemhosseini et al.
[27][49] developed a disposable electrochemical immunosensor for the quantification of HbA1c using a three-electrode system (
Figure 2). A polyethylene terephthalate (PET) sheet served as the flexible substrate for the electrode system, over which the Ag/AgCl reference electrode was printed as a thick film, while gold films deposited by sputtering formed the working and counter electrodes. The gold electrode surface pretreated sequentially in KOH, H
2SO
4, and HNO
3 solutions was modified with a SAM of 3-mercaptopropionic acid (MPA) that served to immobilize anti-HbA1c antibodies via EDC-NHS coupling. The detection of HbA1c using differential pulse voltammetry (DPV) resulted in a linear range of 7.5–20 μg/mL. The sensor was validated in undiluted serum samples, where the linear range changed to 100–250 μg/mL. However, the reported range for diabetic individuals is greater than 9 mg/mL. Therefore, it is evident that the fabricated sensor needs additional modifications to enable detection of HbA1c in the clinically relevant range.
Figure 2. Electrochemical immunosensor based on gold as an interface for direct detection of HbA1c [27]. Electrochemical immunosensor based on gold as an interface for direct detection of HbA1c [49].
In another interesting approach for the quantification of HbA1c using anti-HbA1c IgG antibodies, a mixed layer of oligo (phenyl ethynylene) molecular wires (MW) and an oligo (ethylene glycol) (OEG) were employed as the interface. The distal end of the MW was modified by non-covalent addition of the HbA1c analogue glycosylated pentapeptide (GPP) and the redox mediator 1,1′-di(aminomethyl)ferrocene (FDMA). The sensor used the competitive inhibition assay for the quantification of HbA1c, where the surface-bound GPP and free HbA1c compete for the antibodies in the solution. The binding of the antibodies to the interface resulted in an attenuation in the ferrocene electrochemical response. Higher concentrations of HbA1c resulted in an increase in Faradaic currents due to lesser anti-HbA1c on the electrode interface, and vice versa. The measurement of the change in charge transfer resistance (R
ct) was used for the quantification of the HbA1c between 4.5 and 15.1%
[28][50]. The same group also reported another method to quantify HbA1c based on EIS measurements
[29][51]. In this method, the glassy carbon electrode was modified with 4-aminophenyl (Ph-NH
2) and AuNP. The unreacted amine groups of (Ph-NH
2) were blocked with OEG, followed by surface modification of AuNP with Ph-NH
2, and attachment of GPP. The fabricated sensor upon exposure to anti-HbA1c IgG antibodies resulted in a corresponding change of R
ct of the system due to the selective interaction of anti-HbA1c antibodies and GPP. The sensor had a wide detection range between 0 and 23.3% HbA1c and the values were in agreement with the conventional clinical techniques when tested with human samples. In another variant, a label-free amperometric immunosensor, using a modified interface with the redox mediator FDMA between the GPP- and the Ph-NH
2-modified AuNPs, was also reported for detection of HbA1c based on the competitive immunoassay technique, where the linear detection range was between 4.6 and 15.1% in human blood samples
[30][52].
Ion-sensitive field-effect transistor (ISFET)-integrated chip-based immunosensors have been investigated for quantification of HbA1c
[31][53]. The detection setup used two gold working electrodes, two gold reference electrodes, and a platinum pseudo-reference electrode. The gold reference electrodes were modified by electropolymerization of polypyrrole (PPy), while the working electrodes were first modified by electropolymerization of PPy–HAuCl
4 composite film, followed by in situ deposition of gold nanoparticles. Subsequently, the working electrodes were incubated in diluted antibody solution to immobilize the antibodies on the working electrode. The sensor detected both HbA1c and Hb and the working range of the sensor was between 5 and 20% HbA1c. When the interface was changed to SAM-modified gold nanospheres that were conjugated to mercaptamine-modified gold electrode, followed by immobilization of antibodies to SAMs using EDC-NHS chemistry, the sensor was able to detect HbA1c between the linear range of 50 and 170.5 ng/mL and total Hb between 166.7 and 570 ng/mL
[32][54]. The same strategy was employed to develop a field-effect transistor chip with electrodes surface-modified with 1,6-hexanedithiol and a nano-gold monolayer linked with anti-HbA1c antibodies. The sensor detected Hb and HbA1c between the ranges of 60 and 180 μg/mL and 4 and 24 μg/mL, respectively
[33][55]. The same group had reported a FET-based device where the gold nanofilm was grown by a seed-mediated technique over the gold electrode containing a mixed SAM. After conjugation of the antibodies to the gold nanoparticles, the FET sensor was employed for detection of Hb and HbA1c. The sensor showed a linear range of 1.67–170.5 ng/mL and 16.7–1705 ng/mL for HbA1c and Hb, respectively
[34][56]. The presence of the nanogold layer was found to confer additional sensitivity towards the detection. These studies suggest that apart from the chemical nature, the mode of formation of the interface could also influence the detection range and sensitivity of the resultant sensor.
A modified sandwich immunoassay protocol with an anti-Hb molecule serving as the capture antibody specific to the total Hb, and an enzyme-labeled detection antibody specific for HbA1c, was studied for quantification of HbA1c. Though four different anti-Hb and two different anti-HbA1c antibodies were investigated in the study, none of the results were in agreement with the actual HbA1c values of the samples. It was hypothesized that the detection site of the anti-HbA1c antibody is hidden inside due to folding, and hence an additional denaturation step using 0.2% dodecyltrimethylammonium bromide was introduced in the protocol to expose the antigen-binding site of the anti-HbA1c antibody. This resulted in improved sensitivity of the immunosensor, that was now able to quantify HbA1c between 5.6 and 10.6%
[35][57]. Further tuning of the sensitivity may be required for potential clinical deployment of the sensing device in the future.
Another study employed the sandwich immunoassay technique for dual-detection of HbA1c through electrochemical and optical methods, where the nano-bioprobe was obtained by conjugation of cadmium telluride (CdTe) quantum dots to the secondary antibody. The sandwich immunoassay was performed using an anti-HbA1c antibody as the capture antibody along with the quantum dot-labeled secondary antibody as a dual-mode sensor for the quantification of HbA1c. The sensor employed a small volume of the sample diluted 500 times for the detection and exhibited linearity between 4 and 16% HbA1c
[36][58]. In this line, a bovine serum albumin (BSA)-MWCNT composite crosslinked to a screen-printed carbon electrode by glutaraldehyde was employed as a nano-biosensing interface for the quantification of HbA1c
[24][46]. The anti-HbA1c antibodies were conjugated to the interface along with APBA using EDC-NHS coupling. The resultant immunosensor quantified HbA1c between 2 and 15% in blood samples. The electrode also exhibited good stability over a month when stored in BSA with less than an 8% loss in signal response. Recently, ratiometric studies related to detection of both HbA1c and Hb have been reported
[37][59]. This methodology relied on the sandwich immunoassay, which was assisted by acridine ester-labeling, and the %CV for both repeatability for multiple detections and within-site reproducibility were documented as 1.22 to 2.21% and 2.13 to 3.27%, respectively.