In response to the exhaustion of traditional energy, green and efficient energy conversion has attracted growing attention. The IVA group elements, especially carbon, are widely distributed and stable in the earth’s crust, and have received a lot of attention from scientists. The low-dimensional structures composed of IVA group elements have special energy band structure and electrical properties, which allow them to show more excellent performance in the fields of energy conversion. In recent years, the diversification of synthesis and optimization of properties of IVA group elements low-dimensional nanomaterials (IVA-LD) contributed to the flourishing development of related fields.
In response to the exhaustion of traditional energy, green and efficient energy conversion has attracted growing attention. The IVA group elements, especially carbon, are widely distributed and stable in the earth’s crust, and have received a lot of attention from scientists. The low-dimensional structures composed of IVA group elements have special energy band structure and electrical properties, which allow them to show more excellent performance in the fields of energy conversion. The diversification of synthesis and optimization of properties of IVA group elements low-dimensional nanomaterials (IVA-LD) contributed to the flourishing development of related fields.
- low-dimensional nanomaterials
- IVA group elements
1. Introduction
2. Morphology and Properties of IVA-LD
2.1. 0D Quantum Dots
Nanostructures in different dimensions, such as quantum dots, nanotubes, nanorods/wires, and nanosheets, have provided satisfactory solutions for the rapid development of energy storage and conversion devices, as shown in Figure 1.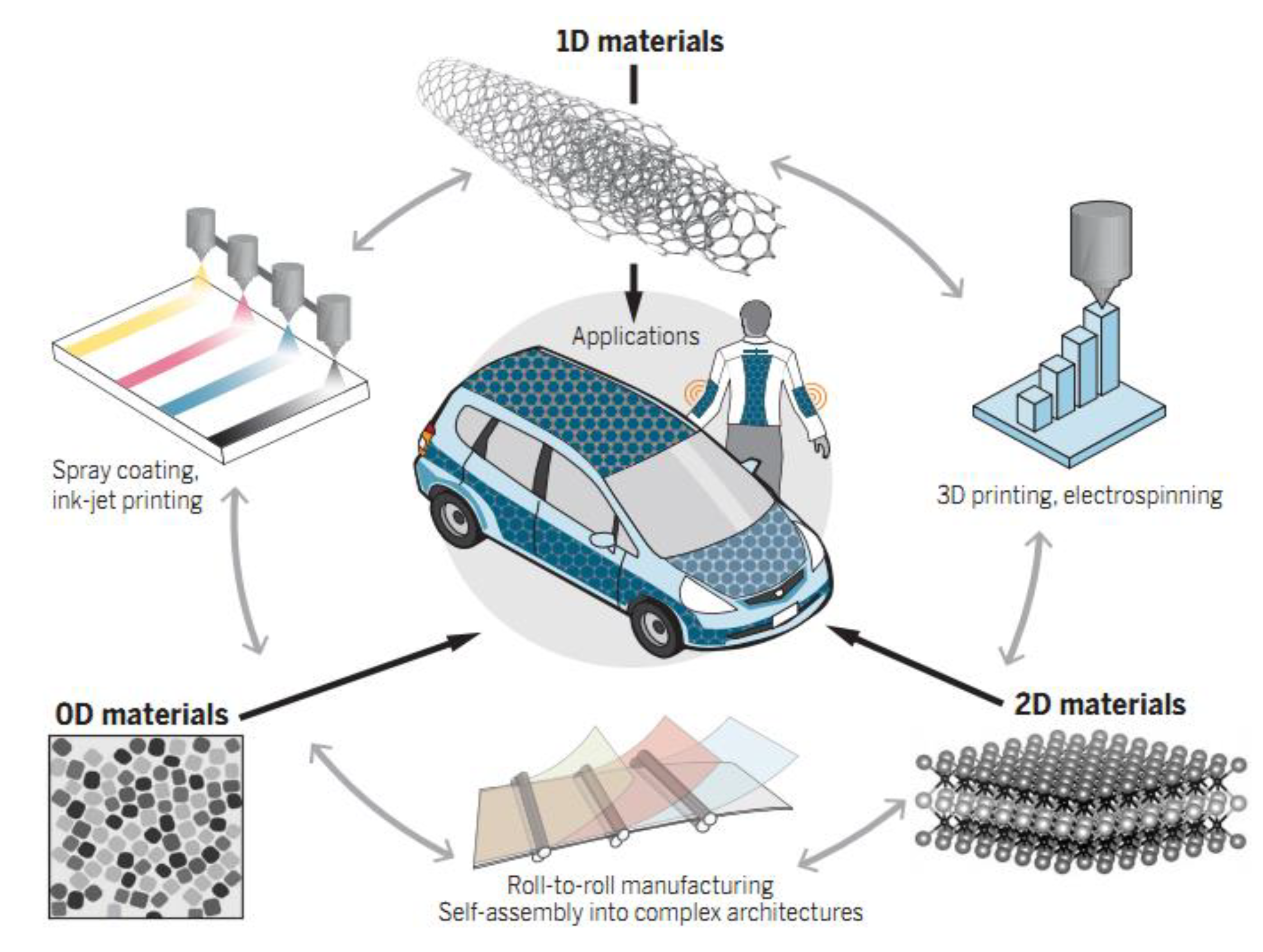
2.2. 1D Nanowires/Rods/Tubes
Nanowires are one-dimensional (1D) structural materials that are laterally confined to less than 100 nm. Compared to conventional bulk materials, nanowires tend to exhibit better photoelectric properties for macroscopic applications. For example, nanowires can naturally concentrate solar energy into a very small area of the crystal, concentrating light 15 times more intensely than ordinary light. This has important implications for the development of solar cells and the use of solar energy [17,18][17][18] because of the resonance of the light intensity within and around the nanowire crystal, which helps to increase the conversion efficiency of solar energy. Silver nanowire electrodes exhibit easily adjustable photoelectric and mechanical properties. Atomic-level chemical welding of silver nanowire electrodes [19] can be used to construct a flexible organic solar cell with high efficiency. Si/InP core-shell nanowire-based solar cell using etched Si nanowire [20] confirm the formation of radial nanowire heterostructures. In this cell, more photons can be absorbed. Compared to traditional solar cells, the performance is greatly improved. CNTs are one of the highest hardness and best strength of synthetic carbon materials. In the CNTs, the C-C bonds are mainly sp2 hybridized, and the hexagonal mesh structure is bent to a certain extent, forming a spatial topology, in which certain sp3 hybridized bonds can be formed. sp2 hybridized C-C bonds are strong chemical bonds, which makes the CNTs have very high mechanical strength. For CNTs with an ideal monolith wall, its tensile strength is about 800 GPa. CNTs are also very flexible and can be stretched. The factor that usually determines the strength of a material is the aspect ratio, the ratio of length to diameter. If the aspect ratio reaches 20, it is an ideal flexible material. CNTs are flexible materials with high thermal conductivity because their aspect ratio can reach more than 1000 and their heat exchange performance along the length direction is very high. CNTs are also divided into single-walled carbon nanotubes (SWCNT) and multi-walled carbon nanotubes (MWCNT). The geometric structure of SWCNT can be regarded as a single layer of graphene crimp, with excellent electronic and mechanical properties. MWCNT is made of layers of graphene seamlessly coiled into concentric tubes. Compared with SWCNT, their elasticity and tensile strength are slightly inadequate. Nanofibers composed of a single polymer often have poor electrical conductivity and weak mechanical properties, so their applications are limited. Therefore, CNTs are often used as reinforcement fillers to prepare nanofibers after compounding with other polymers, which can effectively improve the properties of nanofibers. Fe2O3/C/CNT composites [21] sprayed by ultrasonic can be used as an anode for lithium-ion batteries. In these composites, the high conductivity of CNTs makes charge transfer faster, which improves the performance of lithium-ion batteries. The structure along the CNTs is the same as the sheet structure of graphite, so it also has very good electrical properties. Moreover, it has also realized excellent characteristics in thermal and optical aspects, so it has a very good prospect in battery, sensing, medical treatment, and other aspects. Similarly, silicon and germanium nanotubes are suitable to be used as anodes of lithium-ion batteries because of their cyclic stability. In recent years, further progress has been made on the crystal phase transformation of anode materials during battery charging and discharging, which is expected to improve the performance of lithium-ion batteries. It is worth mentioning that Chen et al. [22] deposited gold (Au), platinum (Pt), nickel (Ni), and indium tin oxide (ITO) onto the surface of tin dioxide nanotubes to prepare different kinds of electrodes. High sensitivity detection of hydrogen and benzene is achieved, and the power consumption is only 1% of that of commercial sensors.2.3. Monolayer
Because of their excellent electronic, optical, and mechanical properties, two-dimensional (2D) nanomaterials graphene, silicene, and germanene have attracted wide interest. Graphene is a new material formed by a single layer of carbon atoms and is also the basic building block of other carbon-based nanomaterials, such as fullerenes, CNTs, and graphite. Graphene can be rolled into 0D fullerenes and 1D CNTs, or it can be stacked in a certain way to form a graphite bulk material. Graphene also exhibits remarkable physical properties due to its unique internal structure. 1. Graphene has excellent electronic properties [23]. It can carry a considerable amount of charged ions, so it is commonly used as a basic raw material for batteries and electrical equipment. 2. Graphene is so flexible that it can bend and fold to a certain extent with little change in its properties. Therefore, graphene has a very good prospect in the research field of flexible wearable electronic devices [24]. But the use of graphene is limited by its lack of a semiconductor band gap. Therefore, it is a difficult problem to study how to open the energy gap. At present, there are two main methods commonly used. The first one is to increase inherent defects of graphene, exposing more active sites. The quantum size effect of the electronic structure can be achieved by changing the morphology of graphene. For example, graphene can be changed into 0D graphene nanoribbons and 0D graphene quantum dots. The second is a chemical modification, which promotes the redistribution of charge on the surface by changing the number and type of heteroatoms incorporated into the graphene. The chemical modification includes surface modification and substitution doping. Graphene surface modification is achieved by hybrid adsorption of gaseous metals or organic molecules on the graphene surface. Alternating doping is the introduction of heteroatoms into the carbon lattice of graphene. At present, this modification method is very mature, and various elements have been widely introduced into graphene. Monoatomic derivatives of graphene can be obtained by adding halogen atoms to the graphene skeleton. Graphene derivatives exhibit different properties due to the different electronegativity of heteroatoms. Among them, fluorographene has large negative magnetoresistance, high optical transparency, and high reactivity. As well, it is easy to generate many derivatives, such as graphene acid and cyanogen graphene. Graphene acid is a novel graphene platform whose carboxylic acid groups are selectively and uniformly located on the surface of the carbon network. This structure enables the graphene acid to have more uniform functionalization and stronger electron conduction. Such good performance also proves the excellent catalytic activity of graphene acid [25]. Furthermore, the selectivity of different oxidation products can be precisely modulated by adjusting the structure of the graphene acid. It is widely used in selective electrochemical sensing and catalysis [26]. Cyano graphene is also one of the graphene derivatives, capable of complex 2D chemical reactions and high yield covalent functionalization of graphene [27]. Since graphene was discovered in 2004, researchers have proposed silicene, germanene, and stanene with graphene-like honeycomb structures. Silicene has since been designed as a cathode to develop zinc-ion hybrid capacitors with enhanced capacitance and hypercyclic stability. As the research progressed, the researchers designed a hybrid honeycomb silicene, combining the electron band gap of specific silicon with the high electron mobility energy of honeycomb silicon. Zhao et al. confirmed that germanene is a potentially high energy density anode material. They prepared small layer germanene nanosheets by the liquid phase stripping method and measured their cyclic stability after mixing with rGO [28]. As mentioned above, wresearchers reviewed the different morphology and properties of IVA-LD. In fact, in research and application, they are not applied alone, but are often used in combination with other low-dimensional or bulk materials, which also shows the advantages of low-dimensional materials for easy composite. The composite of IVA-LD can not only have the excellent properties of each part of the material, but also forms heterojunction at the interface of the composite. Heterogeneous junctions form at the composite interface, among which the Van der Waals heterojunctions (vdWHs) formed by 2D materials stacking has attracted the researchers’ attention for the first time. Some studies show that vdWHs can provide the largest area for the separation and transfer of carriers, showing application potential in photoelectric detection. Some studies have shown that van der Waals heterojunction can provide the largest area for the separation and transfer of carriers, showing the potential of application in photoelectric detection. Dhungana et al. [29] introduced the concept of Xene heterostructures based on an epitaxial combination of silicene and stannene on Ag(111), promising to optimize the responsivity and speed of photodetectors.References
- Kittner, N.; Lill, F.; Kammen, D.M. Energy storage deployment and innovation for the clean energy transition. Nat. Energy 2017, 2, 17125.
- Arent, D.J.; Bragg-Sitton, S.M.; Miller, D.C.; Tarka, T.J.; Garfield, D.J. Multi-input, Multi-output Hybrid Energy Systems. Joule 2020, 5, 47–58.
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22.
- Ajayan, P.M. The nano-revolution spawned by carbon. Nature 2019, 575, 49–50.
- Marrows, C.H. Silicon goes heavyweight. Nat. Mater. 2021, 20, 1177–1178.
- Fadaly, E.M.T.; Dijkstra, A.; Suckert, J.R.; Ziss, D.; van Tilburg, M.A.J.; Mao, C.; Ren, Y.; van Lange, V.T.; Korzun, K.; Kölling, S.; et al. Direct-bandgap emission from hexagonal Ge and SiGe alloys. Nature 2020, 580, 205–209.
- Farquhar, A.K.; Supur, M.; Smith, S.R.; Dyck, C.V.; McCreery, R.L. Hybrid Graphene Ribbon/Carbon Electrodes for High-Performance Energy Storage. Adv. Energy Mater. 2018, 8, 1802439.
- Zhang, X.S.; Kwon, K.; Henriksson, J.; Luo, J.; Wu, M.C. A large-scale microelectromechanical-systems-based silicon photonics LiDAR. Nature 2022, 603, 253–258.
- Luo, W.; Gong, Y.H.; Zhu, Y.Z.; Li, Y.J.; Yao, Y.G.; Zhang, Y.; Fu, K.; Pastel, G.; Lin, C.F.; Mo, Y.J.; et al. Reducing Interfacial Resistance between Garnet-Structured Solid-State Electrolyte and Li-Metal Anode by a Germanium Layer. Adv. Mater. 2017, 29, 1606042.
- Kang, L.T.; Cui, M.W.; Jiang, F.Y.; Gao, Y.F.; Luo, H.J.; Liu, J.J.; Liang, W.; Zhi, C.Y. Nanoporous CaCO3 Coatings Enabled Uniform Zn Stripping/Plating for Long-Life Zinc Rechargeable Aqueous Batteries. Adv. Energy Mater. 2018, 8, 801090.
- Li, Y.Y.; Chen, N.; Li, Z.L.; Shao, H.B.; Sun, X.T.; Liu, F.; Liu, X.T.; Guo, Q.; Qu, L.T. Reborn Three-Dimensional Graphene with Ultrahigh Volumetric Desalination Capacity. Adv. Mater. 2021, 33, 2105853.
- Stetson, C.; Schnabel, M.; Li, Z.F.; Harvey, S.P.; Jiang, C.S.; Norman, A.; Decaluwe, S.C.; Al-Jassim, M.; Burrell, A. Microscopic Observation of Solid Electrolyte Interphase Bilayer Inversion on Silicon Oxide. ACS Energy Lett. 2020, 5, 3567.
- Zhang, C.Y.; Liang, S.X.; Liu, W.; Eickemeyer, F.T.; Cai, X.B.; Zhou, K.; Bian, J.; Zhu, H.W.; Zhu, C.; Wang, N.; et al. Ti1-graphene single-atom material for improved energy level alignment in perovskite solar cells. Nat. Energy 2021, 6, 1154–1163.
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285.
- Li, F.; Li, T.; Sun, C.; Xia, J.; Jiao, Y.; Xu, H. Selenium-Doped Carbon Quantum Dots for Free-Radical Scavenging. Angew. Chem. Int. Ed. 2017, 56, 9910–9914.
- Yu, T.; Wang, F.; Xu, Y.; Ma, L.; Pi, X.; Yang, D. Graphene Coupled with Silicon Quantum Dots for High-Performance Bulk-Silicon-Based Schottky-Junction Photodetectors. Adv. Mater. 2016, 28, 4912–4919.
- Zhang, B.; Jie, J.; Zhang, X.; Ou, X.; Zhang, X. Large-Scale Fabrication of Silicon Nanowires for Solar Energy Applications. ACS Appl. Mater. Interf. 2017, 9, 34527–34543.
- Peng, K.Q.; Lee, S.T. Silicon Nanowires for Photovoltaic Solar Energy Conversion. Adv. Mater. 2011, 23, 198–215.
- Zeng, G.; Chen, W.; Chen, X.; Hu, Y.; Chen, Y.; Zhang, B.; Chen, H.; Sun, W.; Shen, Y.; Li, Y.; et al. Realizing 17.5% Efficiency Flexible Organic Solar Cells via Atomic-Level Chemical Welding of Silver Nanowire Electrodes. J. Am. Chem. Soc. 2022, 144, 8658–8668.
- Pal, B.; Sarkar, K.J.; Banerji, P. Fabrication and studies on Si/InP core-shell nanowire based solar cell using etched Si nanowire arrays. Sol. Energy Mater Sol. Cells 2020, 204, 110217.
- Park, C.; Samuel, E.; Joshi, B.; Kim, T.; Aldalbahi, A.; El-Newehy, M.; Yoon, W.Y.; Yoon, S.S. Supersonically sprayed Fe2O3/C/CNT composites for highly stable Li-ion battery anodes. Chem. Eng. J. 2020, 395, 125018.
- Chen, J.; Chen, Z.; Boussaid, F.; Zhang, D.; Pan, X.; Zhao, H.; Bermak, A.; Tsui, C.Y.; Wang, X.; Fan, Z. Ultra-Low-Power Smart Electronic Nose System Based on Three-Dimensional Tin Oxide Nanotube Arrays. ACS Nano 2018, 12, 6079–6088.
- Ye, M.; Zhang, Z.; Zhao, Y.; Qu, L. Graphene Platforms for Smart Energy Generation and Storage. Joule 2018, 2, 245–268.
- Liang, Y.; Zhao, F.; Cheng, Z.H.; Zhou, Q.H.; Shao, H.B.; Jiang, L.; Qu, L.T. Self-powered wearable graphene fiber for information expression. Nano Energy 2017, 32, 329–335.
- Blanco, M.; Mosconi, D.; Otyepka, M.; Medved, M.; Bakandritsos, A.; Agnoli, S.; Granozzi, G. Combined high degree of carboxylation and electronic conduction in graphene acid sets new limits for metal free catalysis in alcohol oxidation. Chem Sci 2019, 10, 9438–9445.
- Chronopoulos, D.D.; Bakandritsos, A.; Pykal, M.; Zboril, R.; Otyepka, M. Chemistry, properties, and applications of fluorographene. Appl Mater Today 2017, 9, 60–70.
- Bakandritsos, A.; Pykal, M.; Blonski, P.; Jakubec, P.; Chronopoulos, D.D.; Polakova, K.; Georgakilas, V.; Cepe, K.; Tomanec, O.; Ranc, V.; et al. Cyanographene and Graphene Acid: Emerging Derivatives Enabling High-Yield and Selective Functionalization of Graphene. ACS Nano 2017, 11, 2982–2991.
- Zhao, F.; Wang, Y.; Zhang, X.; Liang, X.; Zhang, F.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Few-layer methyl-terminated germanene–graphene nanocomposite with high capacity for stable lithium storage. Carbon 2020, 161, 287–298.
- Dhungana, D.S.; Grazianetti, C.; Martella, C.; Achilli, S.; Fratesi, G.; Molle, A. Two-Dimensional Silicene–Stanene Heterostructures by Epitaxy. Adv. Funct. Mater. 2021, 31, 2102797.
