Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Lindsay Dong and Version 3 by Lindsay Dong.
Carbon capture and storage (CCS) is considered to be a promising technology in reducing atmospheric CO2 concentration. Among the CO2 capture technologies, adsorption has grabbed significant attention owing to its advantageous characteristics discovered in recent years. Solid adsorbents have emerged as one of the most versatile CO2 adsorbents.
- porous carbon
- amine functionalization
- physisorption
- chemisorption
- CO2 capture
- activated carbon
- Greenhouse effect
1. Introduction
1.1. Physical and Chemical Properties of CO2
Carbon dioxide (CO2) is a triatomic gas under ambient conditions [1], which is abundant, non-toxic, recyclable, and economical [2]. Moreover, CO2 sublimates from solid-state to gas at −78 °C under atmospheric pressure and is comparatively inert. As a commonly known fact, CO2 gas that naturally occurs in the Earth’s atmosphere is of paramount importance to photosynthesis [1]. From an economic point of view, CO2 can be converted into high-value chemical products such as urea, carbonates, and acrylates [3] through catalytic conversion, mineralization, photochemical, or electrochemical reactions, and supercritical CO2 can be also utilized in various industrial fields, including food beverages, refrigerants, transportation fuels, fire extinguishers, polymer synthesis, medical, and exploitation of heavy oil. Solid-state CO2 can be used in artificial rainfall and concrete production [4][5].
1.2. Trend of Atmospheric CO2 Concentration and Potential CO2 Emissions Sources
Although the natural carbon cycle controls the CO2 concentration level in the Earth’s atmosphere [1], due to both anthropogenic activities and natural emissions, the current atmospheric CO2 concentration reached around 416.5 ppm in mid-2020 [6], which is ~40% greater than the beginning of the industrial revolution (280 ppm) in 1750 [7][8][9], with an average growth rate of 2 ppm per year [9][10]. In other words, the global emission of CO2 was estimated to be more than 36 MT in 2017, which is 18-fold greater than compared to the 1800s [11]. Although it is a consensus that the amount of atmospheric CO2 should not exceed 350 ppm [12], according to the predictions by the International Panel on Climate Change (IPCC), it is expected to reach up to 570 ppm by 2100 [12][13][14]. It is identified that the main causes for the tremendous increase in such atmospheric CO2 concentration are mainly associated with various anthropogenic activities, including vehicular emissions, fossil-fuel power plants, deforestation, chemical processes [15], and waste treatment [16], which have been growing steadily due to rapid industrialization and urban development [15][17]. The natural emission sources, including soil degradation processes and volcanic activities, are also responsible for supplying atmospheric CO2 to some extent [18].
1.3. Significant Outcomes Owing to the Trend of Increasing CO2 Emissions
Unfortunately, the non-controllable anthropogenic activities have negatively affected human beings [19] and the entire ecosystem [3][6] by releasing greenhouse gases, including CO2, into the atmosphere. Among the greenhouse gases, CO2 is considered as one of the primary sources, contributing to roughly 64% of the total greenhouse effect [14][20]. The progressive increase in atmospheric CO2 concentration is responsible for climate change, which might adversely impact the global environmental processes, such as the long-term rise in global temperatures, changes in rainfall patterns, rising sea levels [21][22], ocean acidification [23], species extinction, melting of polar ice [9], shrinkage of snow covers [24], and severe weather events, ranging from flash floods [25], hurricanes, freezing winters, severe droughts [22], heat waves [26], urban smog [17], and cold streaks [27]. According to the predictions made by IPCC, the rise in sea level of 3.8 m [14][28] and rise in mean global temperature by 3.7 °C [29][30] are expected by 2100 [24]. Besides, the increasing trend of CO2 in the air might cause various air-borne diseases, which will increase the risk of health complications [31]. The economic loss due to climate change is expected to be 5–20% of the global domestic production [12][28]. Therefore, extensive research projects are currently underway to reduce and control CO2 emissions from power plants, industries, and transportation [32].1.4. Approaches to Reduce Atmospheric CO2 Concentration
Three feasible strategies to reduce CO2 emissions are exhibited by the modified Kaya identity as expressed in equation (1) [28]. They are namely, (i) improving the energy efficiency of coal-fired plants [33][34], (ii) change of the fossil fuels to renewable and carbon-free energy resources [35], and (iii) utilization of carbon capture and storage (CCS) technologies [28][36][37].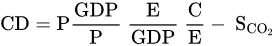 where CD: CO2 emissions, P: Population, GDP: economic development in gross domestic production, E: energy production, C: carbon-based fuels used for energy production, and SCO2: CO2 sinks [28].
Apart from the above-mentioned three strategies, enhancing partial pressure in exhaust gas [36], geoengineering approaches including afforestation and reforestation [38], flue gas separation, and carbon mineralization [39] can also be considered. Among the different CO2 mitigation options, IPCC has suggested CCS as a promising technology for achieving a 19% reduction of global CO2 emissions by 2050 [34]. CCS can reduce CO2 emissions (typically 85–90%) from significant stationary point sources such as power plants, cement kilns, and NG wells [40][41]. Nevertheless, CCS is considered a mid-term solution in reducing global warming, climate change, and simultaneously allowing humans to continue using fossil fuels until a renewable and clean energy source is discovered to replace them [34]. CCS is comprised of three significant steps, namely, (i) capture of emitted CO2 from power plants and industrial processing without releasing them into the atmosphere, (ii) transportation of the captured and compressed CO2, and (iii) underground storage of the captured CO2 [26][42][43]. However, the process of CO2 capture, which accounts for 70–80% of the total cost, has proven to be the major barrier for the deployment of CCS [40][44]. Interestingly, in recent years, carbon capture storage and utilization (CCSU) has grabbed significant attention compared to CCS owing to the convertibility of the captured CO2 into commercial products [45][46]. The success of CCS and CCSU technologies are associated with the CO2 adsorption efficiency, ease of handling, manufacturing cost, and renderability of the associated materials [22].
where CD: CO2 emissions, P: Population, GDP: economic development in gross domestic production, E: energy production, C: carbon-based fuels used for energy production, and SCO2: CO2 sinks [28].
Apart from the above-mentioned three strategies, enhancing partial pressure in exhaust gas [36], geoengineering approaches including afforestation and reforestation [38], flue gas separation, and carbon mineralization [39] can also be considered. Among the different CO2 mitigation options, IPCC has suggested CCS as a promising technology for achieving a 19% reduction of global CO2 emissions by 2050 [34]. CCS can reduce CO2 emissions (typically 85–90%) from significant stationary point sources such as power plants, cement kilns, and NG wells [40][41]. Nevertheless, CCS is considered a mid-term solution in reducing global warming, climate change, and simultaneously allowing humans to continue using fossil fuels until a renewable and clean energy source is discovered to replace them [34]. CCS is comprised of three significant steps, namely, (i) capture of emitted CO2 from power plants and industrial processing without releasing them into the atmosphere, (ii) transportation of the captured and compressed CO2, and (iii) underground storage of the captured CO2 [26][42][43]. However, the process of CO2 capture, which accounts for 70–80% of the total cost, has proven to be the major barrier for the deployment of CCS [40][44]. Interestingly, in recent years, carbon capture storage and utilization (CCSU) has grabbed significant attention compared to CCS owing to the convertibility of the captured CO2 into commercial products [45][46]. The success of CCS and CCSU technologies are associated with the CO2 adsorption efficiency, ease of handling, manufacturing cost, and renderability of the associated materials [22].
1.5. CO2 Emission Sources
The CO2 emission sources are the primary candidates for potential applications of CCS or CCSU technologies. Therefore, from a community and industrial point of view, CO2 capture from typical gas streams, including flue gas, biogas, flare gas, syngas, and ambient air, has grabbed significant interest [47]. Table 1 depicts the summary of the compositions of different gas streams.Table 1. Compositions of different gas streams which act as potential CO2 capture opportunities (Reprinted with permission from ref. [47][48]).
| Component | Cement Rotary Kiln | Dry Atmospheric Air | Biogas Generated from Waste Water Treatment Plant Sludge | Natural Gas Fired Flue Gas | Coal-Fired Flue Gas |
|---|---|---|---|---|---|
| N2 | 59 vol % | 70 vol % | 0–1 vol % | 73–80 vol % | 70–80 vol % |
| CO2 | 19 vol % | 410 ppm | 19–33 vol % | 3–8 vol % | 11–15 vol % |
| H2O | 13 vol % | - | - | 7–14.6 vol % | 5–12 vol % |
| O2 | 7 vol % | 21 vol % | <0.5 vol % | ||
| Kr | |||||
| - | |||||
| 1.1 vol % | |||||
| - | |||||
| - | |||||
| - | |||||
| N2O | - | 0.3 vol % | - | - | - |
1.6. CO2 Capture Technologies
Table 2 depicts the comparison of the leading carbon capture technologies. According to Table 2, carbon capture from power plants in industries can be classified as (i) pre-combustion capture, (ii) oxy-fuel combustion, and (iii) post-combustion capture [49] depending on the combustion method and composition of the gas stream [50]. The working conditions such as pressure and temperature differ for each technique [51]. The main factors impacting CO2 capture efficiency are the gas composition, gas stream temperature, and energy penalty associated with regeneration [28].Table 2. Comparison of the three main carbon capture technologies.
| CO2 Capture Technology | Advantages | Disadvantages | |
|---|---|---|---|
| Pre-combustion capture |
|
|
|
| Chemisorption |
|
|
2.2. Different Regeneration Strategies
The attached CO2 molecules onto the adsorbent surface could be regenerated through the (i) pressure swing adsorption (PSA), (ii) temperature swing adsorption (TSA), (iii) vacuum swing adsorption (VSA), (iv) pressure and vacuum swing adsorption (PVSA), and (v) electric swing adsorption (ESA) processes [26][28][73]. Table 5 shows the advantages and disadvantages of different regeneration strategies. The regeneration method depends on the chemical and structural properties of a given adsorbent [69].Table 4. Comparison of different regeneration strategies.
| Regeneration Strategy | Advantages | Disadvantages | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature swing adsorption (TSA) | |||||||||
| Oxy-fuel combustion |
|
| |||||||
| Pressure swing adsorption (PSA) |
|
|
|
||||||
|
Post-combustion capture | Electric swing adsorption (ESA) |
|
4.5–15 vol % | |||||
| Vacuum swing adsorption (VSA) |
| 3–6 vol % | |||||||
|
|
SO2 | 5–1200 ppm | - | - | <10 ppm | 200–4000 ppm | ||
| SO3 | - | - | - | - | 0–20 ppm | ||||
| NOX | 100–1500 ppm | - | - | 50–70 ppm | 200–800 ppm | ||||
| CO | - | - | - | - |
2.3. Criteria for Selecting CO2 Adsorbents
When synthesizing and selecting an effective CO2 adsorbent, the material should be economical and operational simultaneously [74]. Therefore, a prospective CO2 adsorbent should satisfy the following criteria (Table 5): (i) CO2 adsorption capacity: The adsorption capacity plays a vital role since it determines the amount of adsorbent to be inserted into the adsorption column to attain the desired performance [77][78], (ii) Regenerability: The adsorbent should be fully regenerable and require relatively mild conditions for complete regeneration [78], (iii) CO2 selectivity: The adsorbent should display substantially high selectivity for CO2 in the co-presence of other species (e.g., N2, methane (CH4), sulfur dioxide (SO2), hydrogen sulfide (H2S), and moisture) [74][79][80], (iv) Adsorption/desorption kinetics: A rapid adsorption/desorption is required for swing adsorption to decrease the cycle time [73][74], (v) Thermal, chemical, and mechanical stability: During the cyclic regeneration process, the microstructure and morphology of the adsorbent should be retained. Moreover, the adsorbent should withstand harsh operating conditions, including vibration, high temperatures, pressures, and flow rates. Additionally, the amine-functionalized adsorbents should be resistant against oxidizing agents and contaminants such as sulfur oxides (SOX), nitrogen oxides (NOX), water vapor, and heavy metals [11][81], and (vi) Adsorbent cost: The adsorbent should be synthesized using cheap raw materials while adopting a cost-effective and energy-saving synthesis routes [62].Table 5. Threshold values of criteria for selecting an effective CO2 adsorbent (Reprinted with permission from refs. [74][77]).
| Parameter | Requirement |
|---|
| CO2 adsorption capacity | 3–4 mmol/g | ||||
| Regenerability | >1000 cycles | ||||
| CO2 gas selectivity over other gases | >100 | ||||
| Adsorption/desorption kinetics | >1 mmol/g.min | ||||
| Adsorbent cost | $5–15/kg sorbent | ||||
| 50–100 ppm | |||||
| H2 | - | 0.5 vol % | - | 5–300 ppm | 5–20 g/m3 |
| Particulate matter | - | - | - | - | - |
| H2S | - | - | 100–4000 ppm | - | - |
| Ar | - | 0.9 vol % | - | - | - |
| Xe | - | 0.1 vol % | - | - | - |
| Ne | - | 18 ppm | - | - | - |
| He | - | 5.2 ppm | - | - | - |
| CH4 | - | 1.6 vol % | 60–75 vol % | - | - |
2. Solid Adsorbents for CO2 Capture
2.1. Adsorption Process of CO2
Adsorption is a surface phenomenon that highly depends on surface properties and functionalities [50]. Adsorption of CO2 onto a material occurs through different types of interactions between the gas molecules and the adsorbent. Adsorption can be classified as (i) physisorption or (ii) chemisorption [59]. CO2 adsorption is an exothermic process as reported elsewhere [60][61]. Figure 1 presents the schematic of the two adsorption processes, while Table 3 tabulates the differences between physisorption and chemisorption.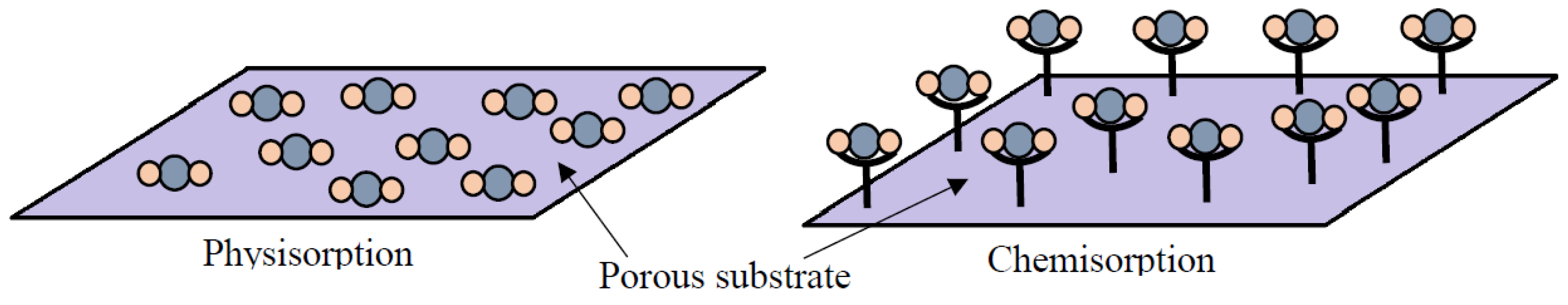
Figure 1. Schematic of the interactions between gas molecules and the adsorbent surface during physisorption and chemisorption (Reprinted with permission from ref. [62]).
Table 3. Comparison of the CO2 physisorption and chemisorption processes.
| Process | Advantages | Disadvantages |
|---|---|---|
| Physisorption | ||
|
||
2.4. Different Adsorbents for CO2 Capture
Numerous studies on CO2 capture conducted in academic and industrial settings have developed promising adsorbents possessing the requirements demonstrated in Table 5 [55]. A variety of adsorbents have been discovered and synthesized, including MOFs, zeolites, activated carbons, zeolite imidazolate frameworks (ZIFs), grafted and impregnated polyamines [44], activated alumina, carbonized porous aromatic frameworks (PAFs), covalent organic frameworks (COFs) [82][83], porous organic polymers (POPs) [33], mesoporous silica, carbon nanotubes [84], metal oxides, ionic liquids [85], phosphates [28], and molecular sieves [5].2.5. Importance of Carbon-Based Adsorbents for Effective CO2 Capture
Of the previously mentioned CO2 adsorbents, though zeolites and well-ordered frameworks exhibit high CO2 adsorption capacities at relatively lower pressures [39], the CO2 adsorption performance gradually decreases in the co-presence of moisture [34][86]. Similarly, molecular sieves and silica gel also demonstrate decreased CO2 adsorption performance in the co-presence of moisture [5]. Additionally, the usage of MOFs has been severely limited due to structural collapse upon vacuum treatments [34], contact with acid gases, thermal regeneration [84], and their complex and expensive synthesis procedures [86]. The ionic liquids are also unfavorable for practical applications due to their relatively high operational costs and high viscosity, leading to corrosion-related problems [87]. On the other hand, the application of carbon materials in the day-to-day lives of human beings can be traced back to more than 5000 years when the early humans discovered charcoal formed through the incomplete combustion of wood. Interestingly, many carbon materials have been discovered, such as graphene, fullerene, activated carbons, graphite, carbon foams, biochar carbon nanotubes, and carbon aerogels [88]. The carbon-based materials can be used as appropriate candidates in catalysis, electronics, fuel cells, biology, metal recovery, and gas storage and separation [27][88][89]. Among the aforementioned wide range of applications, carbon-based porous materials can serve as appropriate candidates for CO2 capture due to their advantageous, including low production cost [27], competitive CO2 adsorption performance at a given pressure [39][90], easy synthesis, ease of scaling up [88], wide availability, controllable pore structure, high thermal stability [15], good chemical resistance against alkaline and acidic media [91], fast adsorption kinetics [44], lower regeneration energy requirements [86], high apparent density (0.3 g/cm3) [92][93], high surface area [94][95], environmental benignity [85], favorable surface chemistry [96], selectivity [66], and flexibility for heteroatom doping or surface functionalization [97]. Additionally, the high thermal and chemical conductivity of carbon-based materials can be exploited for thermal, electric, and pressure swing adsorption strategies [92].References
- North, M. Chapter I-What is CO2? Thermodynamics, basic reactions and physical chemistry. In Carbon Dioxide Utilization; Styring, P., Quadrelli, E.A., Armstrong, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–17.
- Salehi, S.; Anbia, M.; Hosseiny, A.H.; Sepehrian, M. Enhancement of CO2 adsorption of polyethylenimine functionalized multiwalled carbon nanotubes/Cd-nanozeolite composites. J. Mol. Struct. 2018, 1173, 792–800.
- Kaur, B.; Singh, J.; Gupta, R.K.; Bhunia, H. Porous carbons derived from polyethylene terephthalate (PET) waste for CO2 capture studies. J. Environ. Manag. 2019, 242, 68–80.
- Wang, X.; Zhou, J.; Xing, W.; Lui, B.; Zhang, J.; Lin, H.; Cui, H.; Zhou, S. Resorcinol-formaldehyde resin-based porous carbon spheres with high CO2 capture capacities. J. Energy Chem. 2017, 26, 1007–1013.
- Qin, F.; Guo, Z.; Wang, J.; Qu, S.; Zuo, P.; Shen, W. Nitrogen-doped asphaltene-based porous carbon nanosheet for carbon dioxide capture. Appl. Surf. Sci. 2018, 491, 607–615.
- Wang, J.; Yuan, X.; Deng, S.; Zeng, X.; Yu, Z.; Li, S.; Lia, K. Waste polyethylene terephthalate (PET) plastics-derived activated carbon for CO2 capture: A route to a closed carbon loop. Green Chem. 2020, 22, 6836–6845.
- Tehrani, N.H.M.H.; Alivand, M.S.; Maklarany, D.M.; Rashidi, A.; Samipoorgini, M.; Seif, A.; Yousefian, Z. Novel asphaltene-derived nanoporous carbon with N-S-rich-micro-mesoporous structure fro superior gas adsorption: Experimental and DFT study. Chem. Eng. J. 2018, 358, 1126–1138.
- Sepahvand, S.; Jonobi, M.; Ashori, A.; Gauvin, F.; Brouwers, H.J.H.; Oksman, K.; Yu, Q. A promising process to modify cellulose nanotubes for carbon dioxide (CO2) adsorption. Carbohydr. Polym. 2019, 230, 115571.
- Rahimi, K.; Riahi, S.; Abbasi, M.; Fakhroueian, Z. Modification of multi-walled carbon nanotubes by 1,3-diaminepropane to increase CO2 adsorption capacity. J. Environ. Manag. 2019, 242, 81–89.
- Jang, E.; Choi, S.W.; Hong, S.; Shin, S.; Lu, K.B. Development of a cost-effective CO2 adsorbent from petroleum coke via KOH activation. Appl. Surf. Sci. 2017, 429, 62–71.
- Khuong, D.A.; Nguyen, H.N.; Tsubota, T. Activated carbon produced from bamboo and solid residue by CO2 activation utilized as CO2 adsorbents. Biomass Energy 2021, 148, 106039.
- Kaur, B.; Gupta, R.K.; Bhunia, H. CO2 capture on activated carbon from PET (polyethylene terephthalate) waste: Kinetics and modelling studies. Chem. Eng. Commun. 2019, 207, 1031–1047.
- Tiwari, D.; Bhunia, H.; Bajpai, P.K. Development of chemically activated N-enriched carbon adsorbents from urea-formaldehyde resin for CO2 adsorption: Kinetics, isotherm, and thermodynamics. J. Environ. Manag. 2018, 218, 579–592.
- Irani, M.; Jacobson, A.T.; Gasem, K.A.M.; Fan, M. Modified carbon nanotubes/tetraethylpentamine for CO2 capture. Fuel 2017, 206, 10–18.
- Li, Y.; Wang, X.; Cao, M. Three-dimensional porous carbon frameworks derived from mangosteen peel waste as promising materials for CO2 capture and supercapacitors. J. CO2 Util. 2018, 27, 204–216.
- Liu, Z. National carbon emissions from the industry process: Production of glass, soda ash, ammonia, calcium carbide and alumina. Appl. Energy 2016, 66, 239–244.
- Sher, F.; Iqbal, S.Z.; Albazzaz, S.; Ali, U.; Mortari, D.A.; Rashidi, T. Development of biomass derived highly porous fast adsorbents for post-combustion CO2 capture. Fuel 2020, 282, 118506.
- Lal, R. Acceleration soil erosion as a source of atmospheric CO2 soil. Soil Tillage Res. 2019, 199, 35–40.
- Kukulka, W.; Cendrowski, K.; Michalkiewicz, B.; Mkijowska, E. MOF-5 derived carbon as material for CO2 adsorption. R. Soc. Chem. 2019, 9, 18527–18537.
- Dilokekunakul, W.; Teerachawanwong, P.; Klomkliang, N.; Supasitmouskol, S.; Chaemucheun, S. Effects of nitrogen and oxygen functional groups and pore width of activated carbon on carbon dioxide capture: Temperature dependence. Chem. Eng. J. 2020, 389, 124413.
- Arifin, N.P.T.A.; Zulkipils, N.A.N.; Yusof, N.; Ismail, A.F.; Azizi, F.; Salleh, W.N.W.; Jalefar, J.; Nordin, N.A.H.M.; Sazali, N. Preparation and characterization of APTES-functionalized graphene oxide for CO2 adsorption. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 61, 297–305.
- Dassanayake, R.S.; Acharya, S.; Abidi, N. Biopolymer-based material from polysaccharides: Properties, processing, characterization and sorption applications. Adv. Sorpt. Process Appl. 2018, 1–24.
- Li, Y.; Xu, R.; Wang, B.; Wei, J.; Wang, L.; Shen, M.; Yang, J. Enhanced N-doped porous carbon derived from KOH-activated waste wool: A promising material for selective adsorption of CO2/CH4 and CH4/N2. Nanomaterials 2019, 9, 266.
- Omidfar, N.; Mohamadalizadeh, A.; Mousavi, S.H. Carbon dioxide adsorption by modified carbon nanotubes. Asia-Pac. J. Chem. Eng. 2015, 10, 885–892.
- Idrees, M.; Rangari, V.; Jeelani, S. Sustainable packaging waste-derived activated carbon for carbon dioxide capture. J. CO2 Util. 2018, 26, 380–387.
- Lee, S.; Park, S. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11.
- Kamran, U.; Choi, J.R.; Park, S. A role of activation for efficient CO2 affinity on polyacronitrile based porous carbon materials. Front. Chem. 2020, 8, 710.
- Sreenivasalu, B.; Gayatri, D.V.; Sreedhar, I.; Ragharan, K.V. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350.
- Tiwari, D.; Bhunia, H.; Bajpai, P.K. Urea-formaldehyde derived porous carbons for adsorption of CO2. R. Soc. Chem. 2016, 6, 111842–111855.
- Deng, M.; Park, H.G. Spacer-assisted amine-coiled carbon nanotubes for CO2 capture. Langmuir 2019, 35, 4453–4459.
- Jena, K.K.; Panda, A.P.; Verma, S.; Mani, G.K.; Swain, S.K.; Alhassan, S.M. MWCNTs-ZnO-SiO2 mesoporous nano-hybrid materials for CO2 capture. J. Alloy Compd. 2019, 800, 279–285.
- Othman, F.E.C.; Yusof, N.; Ismail, A.F. Activated-carbon nanofibers/graphene nanocomposites and their adsorption performance towards carbon dioxide. Chem. Engl. Technol. 2020, 43, 2023, 2030.
- Shao, L.; Song, Y.; Huang, J.; Liu, Y. Triazine-based hyper-cross-linked polymers with inorganic-organic hybrid framework derived porous carbons for CO2 capture. Chem. Eng. J. 2018, 353, 1–14.
- Salehi, S.; Anbia, M. Highly efficient CO2 capture with a metal-organic framework-derived porous carbon impregnated with polyethylenimine. Appl. Organomet. Chem. 2018, 32, e4390.
- Tiwari, D.; Bhunia, H.; Bajpai, P.K. Adsorption of CO2 on KOH activated N-enriched carbon derived from urea-formaldehyde resin: Kinetics, isotherm and thermodynamic studies. Appl. Surf. Chem. 2018, 439, 760–771.
- Ben-Mansour, R.; Habib, M.A.; Bamidek, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modelling and simulations-a review. Appl. Energy 2016, 161, 225–255.
- Han, J.; Zhang, L.; Zhao, B.; Qin, L.; Wang, Y.; Xing, F. The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption. Ind. Crops Prod. 2019, 128, 290–297.
- Tiwari, D.; Kaur, S.; Bhunia, H.; Bajpai, P.K. CO2 adsorption on oxygen enriched nanostructured carbon derived from silica templated resorcinol-formaldehyde. J. Ind. Eng. Chem. 2018, 65, 146–155.
- Nazir, G.; Rehman, A.; Park, S. Role of heteroatoms (nitrogen and sulfur)-dual doped corn-starch based porous carbons for selective CO2 adsorption and separation. J. CO2 Util. 2021, 51, 101641.
- Benedetti, V.; Cordioli, E.; Patuzzi, F.; Baratieri, M. CO2 adsorption study on pure and chemically activated chars derived from commercial biomass gasifiers. J. CO2 Util. 2019, 33, 46–54.
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbon derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736.
- Gunathilake, C.; Dassanayake, R.S.; Abidi, N.; Jaroniec, M. Amidoxime-functionalized microcrystalline cellulose-mesoprous silica composites for carbon dioxide sorption at elevated temepratures. J. Mater. Chem. A 2016, 4, 4808–4819.
- Alabadi, A.; Razzaue, S.; Yang, Y.; Chen, S.; Tan, B. Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chem. Eng. J. 2015, 281, 606–612.
- Liu, X.; Sun, C.; Liu, H.; Tan, W.H.; Wang, W.; Snape, C. Developing hierarchical ultra-micro mesoporous biocarbons for highly selective carbon dioxide adsorption. Chem. Eng. J. 2018, 361, 199–208.
- Dassanayake, R.S.; Gunathilake, C.; Dassanayake, A.C.; Abidi, N.; Jaroniec, M. Amidoxime-functionalized nanocrystalline cellulose-mesoporous silica composites for carbon dioxide sorption of ambient and elevated temperatures. J. Mater. Chem. A 2017, 5, 7462–7473.
- Dassanayake, R.; Gunathilake, C.; Abidi, N. Activated carbon derived from chitin aerogels: Preparation and CO2 desorption. Cellulose. 2018, 25, 1911–1920.
- Lashake, M.J.; Khiavi, S.; Sayari, A. Stabilizing of amine-functionlaozed CO2 adsorbents: A multifunctional puzzle. Chem. Soc. Rev. 2019, 48, 3320–3405.
- Rashidi, N.A.; Yusup, S. An overview of activated carbon utilization of the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16.
- Zhu, X.; Tsang, D.C.W.; Wang, C.; Su, Z.; Hou, D.; Liangchun, L.; Shang, J. Machine learning exploration of the critical factors for CO2 adsorption capacity on porous carbon materials at different pressures. J. Clean. Prod. 2020, 263, 122915.
- Chatterjee, R.; Sajjadi, B.; Chane, W.; Mattern, D.L.; Hamner, N.; Raman, V.; Dorris, A. Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Front. Energy Res. 2020, 8, 85.
- Singh, J.; Bhunia, H.; Basu, S. Development of sulfur-doped carbon monolith derived from phenol-formaldehyde resin for fixed bed CO2 adsorption. Environ. Innov. 2020, 20, 101104.
- Cueller-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilization technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2014, 9, 82–102.
- Rouzitalab, Z.; Maklavany, D.M.; Jafarinejad, S.; Rashidi, A. Lignocellulose-based adsorbents: A spotlight review of the effective parameters on carbon dioxide capture process. Chemosphere 2020, 246, 125746.
- Yu, J.; Xie, L.H.; Li, J.R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 capture and separations using MOFs: Computational and experimental studies. Chem. Rev. 2017, 117, 9674–9754.
- Wang, P.; Guo, Y.; Zhao, C.; Yan, J.; Lu, P. Biomass derived wood ash with amine modification for post-combustion CO2 capture. Appl. Energy 2017, 201, 34–44.
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63.
- Najmi, B. Operation of Power Cycles with Integrated CO2 Capture Using Advances High-Temperature Technologies; Department of Energy and Process Engineering, Norwegian University of Science and Technology: Trondheim, Norway, 2015; p. 77.
- Zhang, Z.; Borhani, T.N.G.; El-Naas, M.H. Carbon Capture. In Exegetic and Environmental Dimensions; Academic Press, Elsevier: Amsterdam, The Netherlands, 2017.
- Nandi, M.; Uyama, H. Exceptional CO2 adsorbents materials under different conditions. Chem. Rec. 2014, 14, 1134–1148.
- Gadipelli, S.; Patel, A.A.; Guo, Z. An ultrahigh pore volume drives up the amine stability and cyclic CO2 capacity of a sorbent. Adv. Mater. 2015, 27, 4903–4909.
- Chang, B.; Shi, W.; Yin, H.; Zhang, S.; Yang, B. Poplar catkin-derived self-templated synthesis of N-doped hierarchical porous carbon microtubes for efficient CO2 capture. Chem. Eng. J. 2018, 358, 1507–1518.
- Patel, H.A.; Byun, J.; Yavez, C.T. Carbon dioxide capture adsorbents: Chemistry and Methods. ChemSusChem 2017, 10, 1303–1317.
- Kamran, U.; Park, S. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean. Prod. 2021, 290, 125776.
- Chen, Z.; Deng, S.; Wei, H.; Wang, B.; Huang, J.; Yu, G. Activated carbons and amine-modified materials for carbon dioxide capture—A review. Front. Environ. Sci. Eng. 2013, 7, 326–340.
- Tiwari, D.; Geol, C.; Bhunia, H.; Bajpai, P.K. Melamine-formaldehyde derived porous carbons for adsorption of CO2 capture. J. Environ. Manag. 2017, 197, 415–427.
- Geol, C.; Bhunia, H.; Bajpai, P.K. Novel nitrogen enriched porous carbon adsorbents for CO2 capture: Breakthrough adsorption study. J. Environ. Chem. Eng. 2016, 4, 346–356.
- Li, J.; Michalkiewicz, B.; Min, J.; Ma, C.; Chen, X.; Gong, J.; Mijowska, E.; Tang, T. Selective preparation of biomass-derived porous carbon with controllable pore sizes towards highly efficient CO2 capture. Chem. Eng. J. 2018, 360, 250–259.
- Durante, L.A.; Walton, K.S.; Soll, D.S.; Jones, C.W. CO2 capture via adsorption in amine-functionalized sorbents. Curr. Opin. Chem. Eng. 2016, 12, 82–90.
- Wang, M.; Yao, L.; Wang, J.; Zhang, Z.; Qiao, W.; Long, D.; Ling, L. Adsorption and regeneration study of polyethylenemine-impregnated millimeter-sized mesoporous carbon spheres for post-combustion CO2 capture. Appl. Energy 2016, 168, 282–290.
- Shukrullah, S.; Naz, M.Y.; Mohamed, N.M.; Ibrahim, K.A.; Abdel-Salam, N.M.; Ghaffar, A. CVD synthesis, functionalization and CO2 adsorption attribute of multiwalled carbon nanotubes. Processes 2019, 7, 634.
- Faisal, M.; Pamungkas, A.Z.; Krisnandi, Y.K. Study of Amine functionalized mesoporous carbon as CO2 storage materials. Processes 2021, 9, 456.
- Berger, A.H.; Bhown, A.S. Comparing physisorption and chemisorption solid sorbents for use separating CO2 from flue gas using temperature swing adsorption. Energy Proc. 2011, 4, 562–567.
- Xu, C.; Ruan, C.; Li, Y.; Lindh, J.; Stromne, M. High performance activated carbons synthesized from nanocellulose for CO2 capture and extremely selectivity removal of volatile organic compounds. Adv. Sustain. Syst. 2017, 2, 1700147.
- Shi, Y.; Liu, Q.; He, Y. CO2 capture using solid sorbents. In Handbook of Climate Change Mitigation and Adaptation; Springer International Publishing: Cham, Switzerland, 2015.
- Mehrrarz, E.; Ghreyshi, A.A.; Jahanshaki, M. Adsorptive separation of CO2 and CH4 by the broom sorghum based activated carbon functionalized by diethanolamine. Korean J. Chem. Eng. 2016, 34, 413–424.
- Shafeeyan, M.S.; Daud, W.M.A.W.; Shamiri, A.; Aghamohammadi, N. Adsorption equilibrium of carbon dioxide onammonia-modified activated carbon. Chem. Eng. Res. Des. 2015, 104, 42–54.
- Gomez-Pozuelo, G.; Sanz-Perez, E.S.; Arencibia, A.; Pizarro, P.; Sanz, R.; Serrano, D.P. CO2 adsorption on amine-functionalized clays. Microporous Mesoporous Mater. 2019, 282, 38–47.
- Rasoulzadeh, H.; Zarandi, S.M.; Masoundinejad, M.; Amini, M.M. Modelling and optimization by response surface technique for adsorption of carbon dioxide by aminated basilica/alginate composite: Experiments characterization and regeneration studies. Int. J. Environ. Anal. Chem. 2021.
- Sahequi, H.; Galvez, M.E.; Bacatirini, V.; Cheng, Y.; Steinfeld, A.; Zimmermann, T.; Tingant, P. Fast and reversible direct CO2 capture from air onto all-polymer nanofibrillated cellulose-polyethylenimine foams. Environ. Sci. Technol. 2015, 49, 3167–3174.
- Gan, G.; Li, X.; Fan, S.; Wang, L.; Qin, M.; Yin, Z.; Chen, G. Carbon aerogels for environmental clean-up. Eur. J. Inorg. Chem. 2019, 2019, 3126–3141.
- Alveraz-Gutierrez, N.; Gil, M.V.; Rubiera, F.; Peviada, C. Kinetics of CO2 adsorption on cherry stone-based carbons in CO2/CH4 separations. Chem. Eng. J. 2017, 307, 249–257.
- Marin, L.; Dragoi, B.; Olaru, N.; Perju, E.; Coroaba, A.; Doraftei, F.; Scavia, G.; Destri, S.; Zappia, S.; Porzro, W. Nanoporous furfuryl-imine-chitosan fibers as a new pathway towards eco-materials for CO2 adsorption. Eur. Polym. J. 2019, 120, 109214.
- Linga, Z.; Kun, C.; Feng, Z.; Qunfeng, Y. Adsorption of CO2 and H2 on nitrogen-doped porous carbon from Ionic Liquid precursor. Chem. Res. Chin. Univ. 2015, 1, 130–137.
- Ma, X.; Li, L.; Wang, S.; Lu, M.; Li, H.; Ma, W.; Keener, T.C. Ammonia-treated porous carbon derived from ZIF-8 for enhanced CO2 adsorption. Appl. Surf. Sci. 2016, 369, 390–397.
- An, L.; Liu, S.; Wang, L.; Wu, J.; Wu, Z.; Ma, C.; Yu, Q.; Hu, X.C. Novel nitrogen-doped porous carbons derived from graphene for effective CO2 capture. Ind. Eng. Chem. Res. 2019, 58, 3349–3358.
- Yang, M.; Guo, L.; Hu, G.; Hu, X.; Chen, J.; Shen, S.; Dai, W.; Fan, M. Adsorption of CO2 by petroleum coke nitrogen-doped porous carbons synthesized by combining ammoxidation with KOH activation. Am. Chem. Soc. 2016, 55, 757–765.
- Shahrom, M.S.R.; Nordin, A.R.; Wilfred, C.D. The improvement of activated carbon as CO2 adsorbent with supported amine functionalized ionic liquids. J. Environ. Chem. Eng. 2019, 7, 103319.
- Zhao, H.; Luo, X.; Zhang, H.; Sun, N.; Wei, W.; Suo, Y. Carbon-based adsorbents for post-combustion capture: A review. Greenh. Gases Sci. Technol. 2018, 8, 11–36.
- Jayaramulu, K.; Datta, K.K.R.; Shiva, K.; Bhattacharyya, A.J.; Eswaramoortrhy, M.; Maji, T.K. Controlled synthesis of tunable nanoporous carbons for gas storage and supercapacitor application. Microporous Mesoporous Mater. 2015, 206, 127–135.
- Singh, G.; Ismail, I.S.; Bilen, C.; Shanbhas, D.; Sathish, C.I.; Ramadass, K.; Vinu, A. A facile synthesis of activated porous carbon spheres from D-glucose using a non-corrosive activating agent for efficient carbon dioxide capture. Appl. Energy 2019, 255, 113831.
- Psarras, P.; He, J.; Wilcox, J. Effect of water on the CO2 adsorption capacity of amine-functionalized carbon sorbents. Ind. Energy Chem. Res. 2017, 56, 6317–6325.
- Jalilov, A.S.; Li, Y.; Tian, J.; Tour, J.M. Ultra-high surface area activated porous asphalt for CO2 capture through competitive adsorption at high pressures. Adv. Energy Mater. 2016, 7, 1600693.
- Bai, B.C.; Kim, E.A.; Lee, C.W.; Lee, Y.; Im, J.S. Effects of surface chemical properties of activated carbon fibers modified by liquid oxidation for CO2 adsorption. Appl. Surf. Sci. 2015, 353, 158–164.
- Laing, T.; Chen, C.; Li, X.; Zhang, J. Popcorn-derived porous carbon for energy storage and CO2 capture. Langmuir 2016, 32, 8042–8049.
- Chen, S.; Li, Y.; Mi, L. Porous carbons derived from metal organic framework for gas storage and separation: The size effect. Inorg. Chem. Commun. 2020, 118, 107999.
- Ludwinowicz, J.; Jaroniec, M. Potassium salt-assisted synthesis of highly microporous carbon spheres for CO2 adsorption. Carbon 2015, 82, 297–303.
- To, J.W.F.; He, J.; Mei, J.; Haghpanah, R.; Chen, Z.; Kurosuwa, T.; Chen, S.; Bae, W.; Pan, L.; Tok, J.B.H.; et al. Hierarchical N-doped carbon as CO2 adsorbents with high CO2 selectivity from rationally designed polypyrrole precursor. J. Am. Chem. Soc. 2015, 138, 1001–1009.
More
