Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Giovanni Lentini.
The endocannabinoid system (ECS) is one of the most relevant neurotransmitter systems in the brain and plays a pivotal role in the regulation of cognitive abilities, mood, stress, and sleep.
- endocannabinoid system
- FAAH inhibitors
- MGL inhibitors
- repositioning
1. Introduction
The endocannabinoid system (ECS) is one of the most relevant neurotransmitter systems in the brain and plays a pivotal role in the regulation of cognitive abilities, mood, stress, and sleep [13][1]. Relatively fewer explored targets for antipsychotic treatment could be found in ECS. Thinking about cannabinoids, the minds run to the well-known pro-psychotic properties of ∆9-tetrahydrocannabinol (THC, Figure 1), the main psychoactive ingredient of cannabis, which acts as an agonist on cannabinoid (CB) receptors (CBRs). In addition to its pro-psychotic potential, THC causes an undesirable behavioral tetrad, that is, analgesia, catalepsy, hypothermia, and hypolocomotion. THC synthetic analogs, both agonists and antagonists [14,15][2][3], or recreational drugs—the so-called NPS (new psychoactive substances) [16,17][4][5]—are generally tainted with severe side effects. The worst is that the activation of CBR of type 1 (CB1R) in the central nervous system (CNS) by xenobiotics can lead to irreversible effects [18][6]. On the other hand, (−)-trans-cannabidiol (CBD, Figure 1), one of cannabis’ main secondary metabolites, seems to be endowed with antipsychotic properties useful to protect against the pro-psychotic effects of THC: depending on its composition, cannabis would act either as Mister Hyde (i.e., a risk factor for psychosis) or as Doctor Jekyll (i.e., an antipsychotic). The hypothesis has been formulated that CBD could be an antipsychotic, with benefits in preventing psychotic disorders, whatever the cause (endogenous or THC-induced) [19][7].
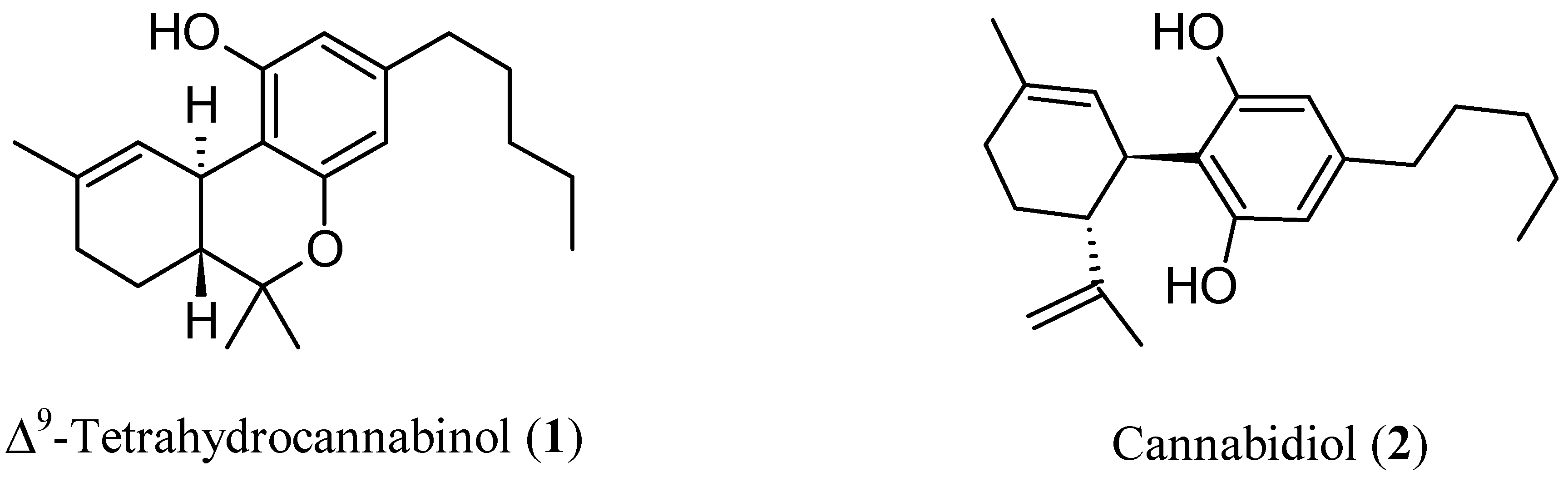
Figure 1.
Chemical structures of Δ
9
-tetrahydrocannabinol (THC,
1
) and cannabidiol (CBD,
2
).
In a randomized, double-blind controlled clinical trial, CBD exerted antipsychotic properties comparable to the reference drug amisulpride [20,21][8][9]. Interestingly, the reduction of psychotic symptoms was significantly associated with an increase in the serum concentrations of N-arachidonoylethanolamine (anandamide, AEA, Figure 2), which is the most important endogenous ligand of CBR, and this outcome was found only in patients treated with CBD. The results indicated that, at least in part, the antipsychotic activity of CBD was due to the inhibition of the enzymes physiologically devoted to the degradation of AEA [22][10], thus acting as an indirect agonist. This finding agrees with the observation that both increased availability of CB1R and upregulation of AEA seem beneficial, although the underlying mechanisms are mostly elusive. The evidence supporting the possible protective role of AEA in schizophrenia has been reviewed [23][11].
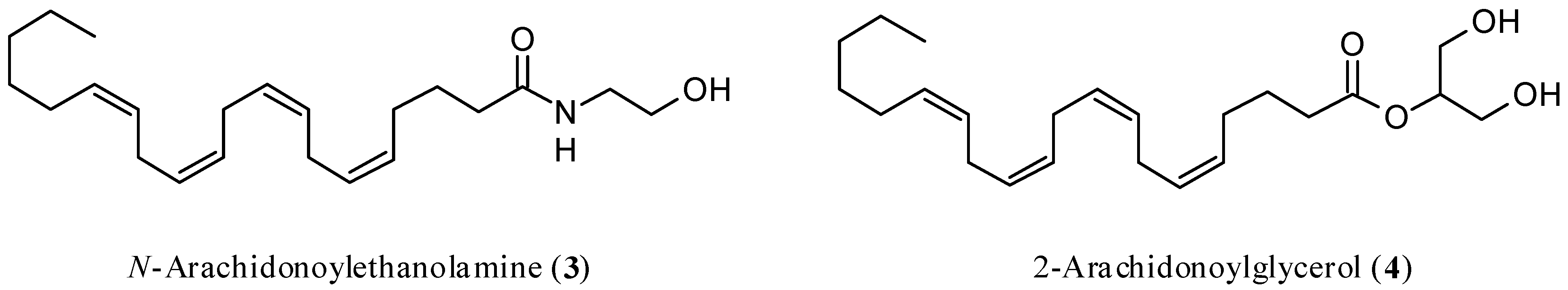
Figure 2.
Chemical structures of
N
-arachidonoylethanolamine (anandamide, AEA,
3
) and 2-arachidonoylglycerol (2-AG,
4
).
The inhibition of enzymes responsible for the degradation of the ECS endogenous ligands might overcome the above obstacles to the systemic use of exogenous substances acting on ECS. EC degradation inhibitors would provide focused action, where and when necessary, by acting as endogenous CBRs ligand modulators.
2. Endocannabinoid System
The exhaustive description of ECS architecture and functioning is beyond the scope of this work; more details can be found elsewhere [24,25][12][13]. The right functioning of the ECS is related to the natural balance established between its main components, which are CBRs, the endogenous ligands binding them, and the enzymes involved in the synthesis, transport, and degradation of ECs. A disruption of the physiological activity of this system (i.e., modifications in the expression of receptors or the functions of enzymes) is associated with various pathologies. This situation, therefore, is the basis for therapeutic pharmacological opportunities founded on drugs able to interact naturally with ECS [26,27,28,29,30][14][15][16][17][18].
The discovery of CBRs and the main endogenous ligands is relatively recent, as the first one, CB1R, was identified in the second half of the 1980s [31][19], while the second receptor, namely CB2R, was discovered a few years later [32][20]. The two targets differ in their corresponding main functions, signaling processes, and structural aspects [33,34][21][22]. Their signal neurobiology and tissue distribution are also different, being the CB1R mainly expressed in the CNS (mostly in the basal ganglia, cerebellum, cortex, and hippocampus), whereas CB2R is particularly present in the immune system (mostly in B-cells and natural killers) [35][23]. Overall, it is demonstrated that CBR, through their activation, performs a key role in inducing activation or depression of neurotransmission by the inhibition of adenylate cyclase, which determines a decrease in cyclic adenosine monophosphate levels, or, only in the case of CB1R, by the coupling with ion channels [35,36][23][24]. A careful analysis of the above characteristics, in particular those related to the different tissue distribution, is important when envisioning a pharmacological therapy aiming at a selectivity of action and the consequent reduction of undesired effects.
The main and most studied CBR endogenous ligands are AEA [37][25] and 2-arachidonoylglycerol (2-AG) [38,39][26][27] (Figure 2).
Both ligands are produced on demand from membrane phospholipids to satisfy contingent physiological needs due to intense neuronal activation [40,41][28][29]. AEA and 2-AG act through a retrograde or non-retrograde signaling pathway. Their half-life is short (a few minutes) as a rapid carrier-mediated diffusion occurs in the cells where they are metabolized [40,41][28][29]. It is very interesting to consider that ECs-mediated retrograde signaling is involved in the excitatory or inhibitory processes related to the modulation of neurotransmitters, such as glutamate or γ-aminobutyric acid [41[29][30][31][32],42,43,44], through short-term and long-term neuroplasticity (taking some seconds and some minutes, respectively) physiological processes [45,46][33][34]. The first is involved in processes such as depolarization-induced suppression of inhibition and depolarization-induced suppression of excitation through the inhibition of voltage-gated Ca2+ channels, whereas the second one leads to the long-term depression phenomenon through a CB1R repeated stimulation of these brain circuits. Consequently, CBR has to be considered a potential drug target for the prevention and treatment of neurologic pathologies, in particular, in the case of CNS involvement [47][35].
AEA is biosynthesized by the N-acyl phosphatidylethanol-selective phospholipase D [48][36]. It acts as a total or partial agonist of the CB1R and has low activity toward CB2R [49][37]. AEA comes up against rapid degradation due to its capture by a transporter [50[38][39],51], as occurs in the extracellular space of brain neurons and astrocytes [29][17], which is followed by the degradative action mainly carried out by fatty acid amide hydrolase (FAAH) [52,53,54[40][41][42][43],55], a homodimer integral membrane protein. The functional component of the enzyme consists of a catalytic triad formed by the amino acids Lys142-Ser217-Ser241, with the latter determining the nucleophilic attack on the electrophilic carbonyl group of AEA through the hydroxy group [56][44].
The biosynthesis of 2-AG begins with diacylglycerols and hydrolysis operated by the diacylglycerol lipase isoform α or β [57,58][45][46]. It acts as a full agonist of both CB1R and CB2R [59][47]. In addition, in the case of 2-AG, therefore, the molecule is captured by a transporter with characteristics identical or similar to those shown by AEA [60][48], which causes internalization and subsequent hydrolysis mainly by monoacylglycerol lipase (MGL) [61[49][50][51],62,63], an enzyme belonging to the α/β hydrolase superfamily. The mechanism involves the participation of various amino acids, which contribute to the initial preparatory phase aimed at catalytic activity by the Ser122-Asp239-His269 triad, where the serine residue is responsible for the nucleophilic action towards the carbonyl group of the substrate [64,65][52][53].
References
- Graczyk, M.; Łukowicz, M.; Dzierzanowski, T. Prospects for the use of cannabinoids in psychiatric disorders. Front. Psychiatry 2021, 12, 620073.
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting cannabinoid receptors: Current status and prospects of natural products. Int. J. Mol. Sci. 2020, 21, 5064.
- Deventer, M.H.; Van Uytfanghe, K.; Vinckier, I.M.J.; Reniero, F.; Guillou, C.; Stove, C.P. Cannabinoid receptor activation potential of the next generation, generic ban evading OXIZID synthetic cannabinoid receptor agonists. Drug Test. Anal. 2022, 14, 1565–1575.
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197.
- Markham, J.; Sparkes, E.; Boyd, R.; Chen, S.; Manning, J.J.; Finlay, D.; Lai, F.; McGregor, E.; Maloney, C.J.; Gerona, R.R.; et al. Defining steric requirements at CB1 and CB2 cannabinoid receptors using synthetic cannabinoid receptor agonists 5F-AB-PINACA, 5F-ADB-PINACA, PX-1, PX-2, NNL-1, and their analogues. ACS Chem. Neurosci. 2022, 13, 1281–1295.
- Chang, H.-A.; Dai, W.; Hu, S.S.-J. Sex differences in cocaine-associated memory: The interplay between CB1, mGluR5, and estradiol. Psychoneuroendocrinology 2021, 133, 105366.
- Morrison, P.D.; Murray, R.M. The antipsychotic landscape: Dopamine and beyond. Ther. Adv. Psychopharmacol. 2018, 8, 127–135.
- Leweke, F.M. Anandamide dysfunction in prodromal and established psychosis. Curr. Pharm. Des. 2012, 18, 5188–5193.
- Rohleder, C.; Müller, J.K.; Lange, B.; Leweke, F.M. Cannabidiol as a potential new type of an antipsychotic. A critical review of the evidence. Front. Pharmacol. 2016, 7, 422.
- Criscuolo, E.; De Sciscio, M.L.; Fezza, F.; Maccarrone, M. In silico and in vitro analysis of major cannabis-derived compounds as fatty acid amide hydrolase inhibitors. Molecules 2021, 26, 48.
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94.
- Maccarrone, M. Missing pieces to the endocannabinoid puzzle. Trends Mol. Med. 2020, 26, 263–272.
- Duranti, A.; Beldarrain, G.; Álvarez, A.; Sbriscia, M.; Carloni, S.; Balduini, W.; Alonso-Alconada, D. The endocannabinoid system as a target for neuroprotection/neuroregeneration in perinatal hypoxic–ischemic brain injury. Biomedicines 2023, 11, 28.
- Mangiatordi, G.F.; Intranuovo, F.; Delre, P.; Abatematteo, F.S.; Abate, C.; Niso, M.; Creanza, T.M.; Ancona, N.; Stefanachi, A.; Contino, M. Cannabinoid receptor subtype 2 (CB2R) in a multitarget approach: Perspective of an innovative strategy in cancer and neurodegeneration. J. Med. Chem. 2020, 63, 14448–14469.
- Ren, S.; Wang, Z.; Zhang, Y.; Chen, N. Potential application of endocannabinoid system agents in neuropsychiatric and neurodegenerative diseases—Focusing on FAAH/MAGL inhibitors. Acta Pharmacol. Sin. 2020, 41, 1263–1271.
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The endocannabinoid system: A potential target for the treatment of various diseases. Int. J. Mol. Sci. 2021, 22, 9472.
- Piomelli, D.; Mabou Tagne, A. Endocannabinoid-based therapies. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 483–507.
- Intranuovo, F.; Brunetti, L.; DelRe, P.; Mangiatordi, G.F.; Stefanachi, A.; Laghezza, A.; Niso, M.; Leonetti, F.; Loiodice, F.; Ligresti, A.; et al. Development of N-(1-adamantyl)benzamides as novel anti-inflammatory multitarget agents acting as dual modulators of the cannabinoid CB2 receptor and fatty acid amide hydrolase. J. Med. Chem. 2022, 66, 235–250.
- Devane, W.A.; Dysarz, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613.
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65.
- Shahbazi, F.; Grandi, V.; Banerjee, A.; Trant, J.F. Cannabinoids and cannabinoid receptors: The story so far. IScience 2020, 23, 101301.
- Tang, X.; Liu, Z.; Li, X.; Wang, J.; Li, L. Cannabinoid receptors in myocardial injury: A brother born to rival. Int. J. Mol. Sci. 2021, 22, 6886.
- Lutz, B. Neurobiology of cannabinoid receptor signaling. Dialogues Clin. Neurosci. 2020, 22, 207–222.
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Ma, A.L.; Mitchell, R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995, 48, 443–450.
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949.
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90.
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylgylcerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97.
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884.
- Augustin, S.M.; Lovinger, D.M. Functional Relevance of endocannabinoid-dependent synaptic plasticity in the central nervous system. ACS Chem. Neurosci. 2018, 9, 2146–2161.
- Diana, M.A.; Marty, A. Endocannabinoid-mediated short-term synaptic plasticity: Depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br. J. Pharmacol. 2004, 142, 9–19.
- Hohmann, A.G.; Suplita, R.L.; Bolton, N.M.; Neely, M.H.; Fegley, D.; Mangieri, R.; Krey, J.F.; Walker, J.M.; Holmes, P.V.; Crystal, J.D.; et al. An endocannabinoid mechanism for stress-induced analgesia. Nature 2005, 435, 1108–1112.
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and synaptic function. Neuron 2012, 76, 70–81.
- Alger, B.E. Retrograde Signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog. Neurobiol. 2002, 68, 247–286.
- Makara, J.K.; Mor, M.; Fegley, D.; Szabó, S.I.; Kathuria, S.; Astarita, G.; Duranti, A.; Tontini, A.; Tarzia, G.; Rivara, S.; et al. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat. Neurosci. 2005, 8, 1139–1141.
- Estrada, J.A.; Contreras, I. Endocannabinoid receptors in the CNS: Potential drug targets for the prevention and treatment of neurologic and psychiatric disorders. Curr. Neuropharmacol. 2020, 18, 769–787.
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372, 686–691.
- Hillard, C.J.; Campbell, W.B. Biochemistry and pharmacology of arachidonylethanolamide, a putative endogenous cannabinoid. J. Lipid Res. 1997, 38, 2383–2398.
- Hillard, C.J.; Edgemond, W.S.; Jarrahian, A.; Campbell, W.B. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J. Neurochem. 1997, 69, 631–638.
- Beltramo, M.; Stella, N.; Calignano, A.; Lin, S.Y.; Makriyannis, A.; Piomelli, D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 1997, 277, 1094–1097.
- Desarnaud, F.; Cadas, H.; Piomelli, D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J. Biol. Chem. 1995, 270, 6030–6035.
- Hillard, C.J.; Wilkison, D.M.; Edgemond, W.S.; Campbell, W.B. Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochim. Biophys. Acta 1995, 1257, 249–256.
- Ueda, N.; Kurahashi, Y.; Yamamoto, S.; Tokunaga, T. Partial purification and characterization of the porcine brain enzyme hydrolyzing and synthesizing anandamide. J. Biol. Chem. 1995, 270, 23823–23827.
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87.
- Patricelli, M.P.; Cravatt, B.F. Clarifying the catalytic roles of conserved residues in the amidase signature family. J. Biol. Chem. 2000, 275, 19177–19184.
- Farooqui, A.A.; Rammohan, K.W.; Horrocks, L.A. Isolation, characterization, and regulation of diacylglycerol lipases from the bovine brain. Ann. N. Y. Acad. Sci. 1989, 559, 25–36.
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.-J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003, 163, 463–468.
- Sugiura, T.; Waku, K. 2-Arachidonoylglycerol and the cannabinoid receptors. Chem. Phys. Lipids 2000, 108, 89–106.
- Beltramo, M.; Piomelli, D. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonylglycerol. NeuroReport 2000, 11, 1231–1235.
- Tornqvist, H.; Belfrage, P. Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J. Biol. Chem. 1976, 251, 813–819.
- Prescott, S.M.; Majerus, P.W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J. Biol. Chem. 1983, 258, 764–769.
- Farooqui, A.A.; Taylor, W.A.; Horrocks, L.A. Separation of bovine brain mono- and diacylglycerol lipases by heparin sepharose affinity chromatography. Biochem. Biophys. Res. Commun. 1984, 122, 1241–1246.
- Karlsson, M.; Contreras, J.A.; Hellman, U.; Tornqvist, H.; Holm, C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J. Biol. Chem. 1997, 272, 27218–27223.
- Labar, G.; Bauvois, C.; Borel, F.; Ferrer, J.-L.; Wouters, J.; Lambert, D.M. Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. ChemBioChem 2010, 11, 218–227.
- van Egmond, N.; Straub, V.M.; van der Stelt, M. Targeting endocannabinoid signaling: FAAH and MAG lipase inhibitors. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 441–463.
More
