Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Hyrije Vesel Koraqi.
The scientific name of citrus is
Citrus
L. and it belongs to the
Rutaceae
family. It is one of the most important fruit crops that is grown throughout the world. During processing, a large amount of waste is produced from citrus fruits in the form of peel, seeds, and pomace. Every year, the citrus processing industry creates a large amount of waste. The citrus waste is composed of highly bioactive substances and phytochemicals, including essential oils (EOs), ascorbic acid, sugars, carotenoids, flavonoids, dietary fiber, polyphenols, and a range of trace elements.
- citrus waste
- potential health benefits
- bioactive compounds
1. Introduction
Citrus is one of the most important fruit crops in the world. The scientific name of citrus is Citrus L. and it is a member of the Rutaceae family. It is grown extensively in areas that are classified as tropical or subtropical, as well as many other areas, which together produce more than 100 million tons annually [1]. Customers display a significant preference for citrus fruits because of their attractive colors, flavorful aromas, and pleasant flavors. Worldwide, citrus fruits are most commonly cultivated crops. Citrus fruits are an important staple food in the diets of people all over the world [2]. These fruits are crucial throughout the world both nutritionally and commercially [3]. Citrus species could be a source of valuable oils that could be used in food and for other industrial purposes [4]. Around the world, citrus fruits are considered nutrient-dense, energy-dense, and health-promoting fruits. Several of these fruits (lemon, grapefruit, sweet orange, citron, clementine, and pomelo) have also been used as traditional medicinal herbs in Asian countries to treat a variety of illnesses. Several studies showed that citrus fruits contained secondary metabolites as well as bioactive compounds that can be used as either chemotherapeutics or supplements [5,6][5][6]. The secondary metabolites found in citrus fruits are important to human health due to their functional properties. There are different secondary metabolites present in citrus waste, including coumarins, alkaloids, carotenoids, limonoids, phenolic acids, flavonoids, and essential oils (EOs) [7]. The utilization of plant residues can reduce the risk of metabolic syndrome-related ailments such as neurodegenerative diseases, diabetes, cardiovascular disease, and cancer. Citrus L. is a basic fruit crop that contains high levels of flavonoids, carotenoids, limonoids, terpenes, and other bioactive components [8]. Citrus fruits are abundant in vitamins C, A, and E, minerals, coumarins, flavonoids, limonoids, pectins, carotenoids, and other beneficial phytochemicals. The phytochemicals are consumed in the form of fresh fruits or products and exert positive effects on people’s health, including anti-mutagenicity, antioxidant, anti-carcinogenic, anti-inflammatory, and anti-aging effects [9]. Additionally, these phytochemicals promote cardiovascular health and nervous system function [7]. Citrus fruit phytochemicals may have antioxidant properties via raising liver protective enzyme activity, blocking lipids to prevent deoxyribonucleic acid (DNA) damage, and bolstering the immune system [10]. Citrus fruits are prized for their nutritional content and for providing some of the world’s most popular flavors. Growing citrus fruits (tangerines, lemons, oranges, grapefruits, and limes) is one of the most common means of producing fruit across the globe. Due to expanding customer demands, their output is increasing year after year [11]. Every year, the citrus processing industry produces a large amount of fruit waste, with citrus peel waste accounting for over half of the wet fruit mass. The waste from citrus has fundamental economic value because it contains a great deal of EOs, ascorbic acid, sugars, carotenoids, flavonoids, dietary fiber, polyphenols, and trace elements. This waste also includes a high concentration of sugars that can be fermented to produce bioethanol. These components are useful in the production of medicines, cosmetics, and food supplements [12].
2. Different Sources of Citrus Waste
Oranges, lemons, grapefruits, mandarins, and limes are the citrus fruits that are most commonly grown around the world. Due to escalating consumer demands, production grows yearly. Annually, the industries that process citrus produce large amounts of waste, with citrus peel waste alone representing nearly 50% of the wet fruit mass [13]. Food waste is composed of ingredients that are useful for human utilization, but these are degraded, polluted, and discarded. Food waste is an increasing issue that affects all facets of waste management. In order to develop long-term solutions, all actors involved in the food supply chain, including the industry, agriculture, merchants, collection to disposal processes, and consumers, must participate [14]. The processing of citrus fruit generates a range of waste, including liquid, solid, and distillery effluents. Rags, peels, sludge, seeds, and residue constitute solid waste. However, there are different types of liquid waste too, including cannery effluents, can cooler overflows, fruit washing wastewaters, sectioning table and peeling wastewaters, and floor flushing water [15]. Citrus is primarily used in the food manufacturing process to create fresh juice or drinks with citrus flavors, resulting in a significant amount of citrus waste each year in the form of peels, pulp, and seeds. However, the seeds, pulp, and peels of many fruits and vegetables may contain a significant number of bioactive compounds [16]. A previous study was conducted on the recovery of vitamin C, phenolic compounds, and antioxidant activity from citrus (lemon, orange, and grapefruit) fruit waste. Each citrus fruit’s peel, whole fruit, and pulp with seeds were converted into ethanol extracts. Results showed that peels had higher levels of phenolic compounds, vitamin C, flavonoids, and antioxidant activity than their internal, wasted parts (seeds and pulp) in each citrus variety [17]. The extracts (peel, pulp, and seeds) of Citrus reticulata (Phlegraean mandarin), Citrus japonica (Kumquat), and Citrus clementina were compared and characterized in terms of photosynthetic pigment content, total polyphenol amount, antioxidant activity, and vitamin C [18]. The generation of waste from citrus processing is shown in Figure 1.
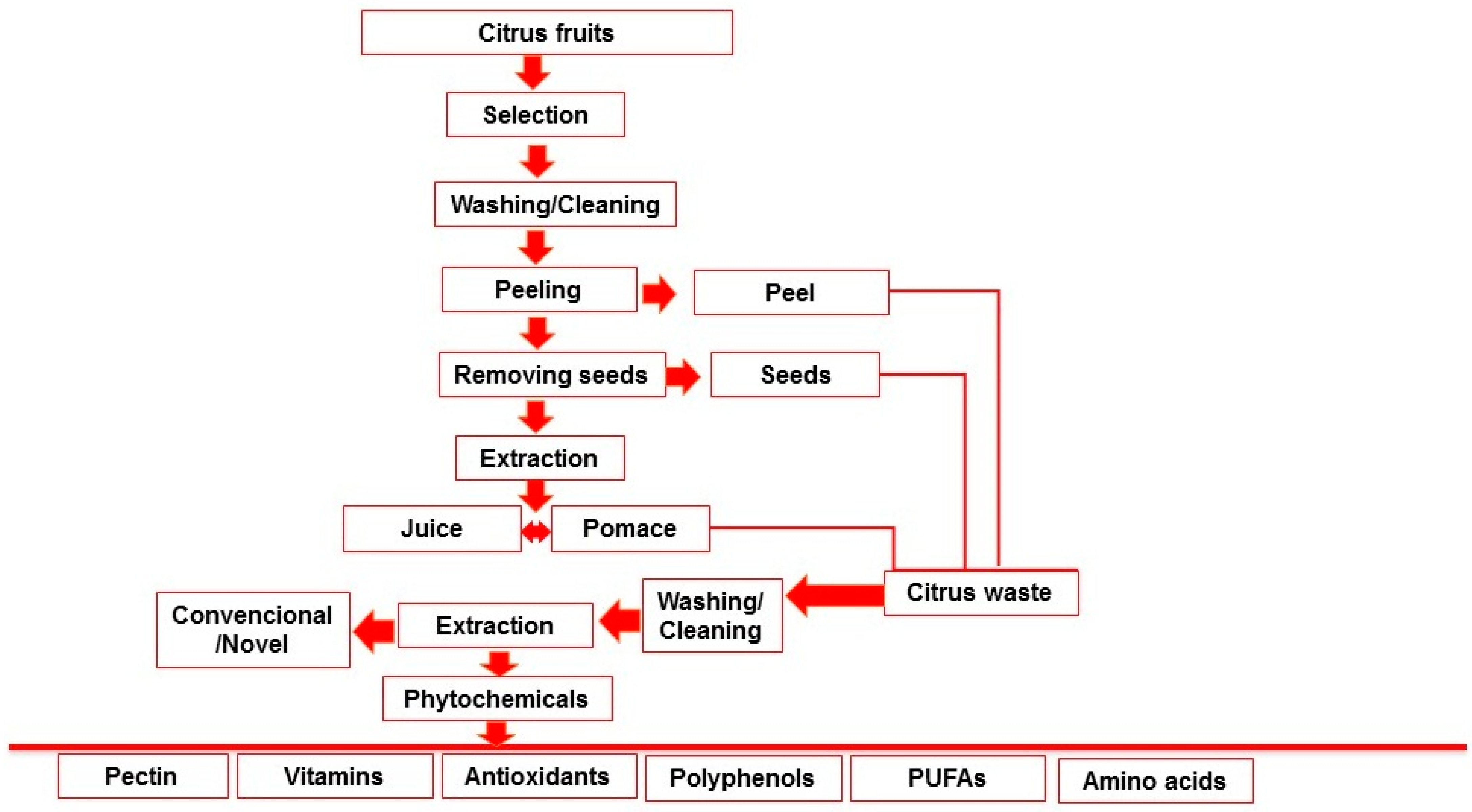
Figure 1. Generation of waste in juice processing.
2.1. Citrus Peel
In the quest to reduce global food losses and waste, citrus peel waste (CPW) in particular has emerged as a sustainable and promising option for biorefinery without competing with human foods and animal feeds. According to recent studies, CPW is widely produced and has the industrial potential to be biologically converted into fuels and chemicals [19]. The extraction of essential oils (limonene) and pectin for use as cosmetic and food additive ingredients is the most widely known method for using CPW [20]. Fruit waste is produced in large quantities by agricultural processes all over the world. Frequently, this waste is simply discarded into landfills or the ocean. Fruit waste contains a variety of sugars, such as fructose, sucrose, and glucose, which can be fermented to produce bioethanol. Some fruit wastes, such as citrus peel waste (CPW), contain substances that can stop fermentation, which is necessary for efficient bioethanol production. A novel method for converting CPW from a single source (mandarin, orange, lemon, grapefruit, or lime) or CPW combined with other fruit waste (apple pomace, banana peel, or pear waste) into bioethanol has been developed [21].
2.2. Citrus Seeds
After the seeds are gathered, cleaned, dried, and ground, it is necessary to optimize a number of downstream processes in order to extract phytoconstituents from them. Solvents, time, temperature, pressure, and particle size are some of the steps involved in downstream processes. Various extraction techniques are used for citrus seeds, including supercritical CO2 extraction, solvent extraction, cold pressing, and ultrasound-assisted extraction [22]. Citrus seeds are typically discarded as waste because they are thought to be useless. According to Ammerman and Arrington [23], the average percentage of seeds in dried citrus pulp is 4.8%. These discarded seeds can be used profitably as a protein supplement for livestock, because they are high in protein. Furthermore, citrus seeds have high potential for use as biodiesel due to their 30% oil content (by weight). According to a rough analysis of flour produced from unhulled and dehulled citrus seeds, it contained 28.5% carbohydrates, 52% fat, 3.1% crude protein, 5.5% crude fiber, and 2.5% ash (dry basis). Rashid et al. [24] produced methyl esters that complied with both the ASTM D6751 and EN 14214 biodiesel standards by trans-esterifying citrus seed oil with methanol under the catalysis of sodium methoxide.
2.3. Citrus Pomace
Citrus pomace is the leftover residue from the processing of citrus fruit to create juice or other products. When citrus fruits are processed into industrial citrus juice, nearly half of their volume is wasted, and the enormous amount of citrus pomace causes serious ecological problems [25]. In the case of not using adequate citrus, the pomace generated by the agro-fruit industry has a negative impact on the environment and will result in significant financial losses. Fruit pomaces are rich in a variety of beneficial bioactive substances, including dietary fiber, carbohydrates, phenolic compounds, polysaccharides, phytochemicals, natural antioxidants, and a number of other nutrients that are beneficial for health [26].
3. Extraction of Bioactive Compounds from Citrus Waste
Culturing citrus fruits requires extensive processing. However, after processing, a number of by-products and wastes are produced that are rich sources of bioactive components, such as pectin, essential oils (EOs), and water-insoluble and -soluble antioxidants. The major phytochemicals of Citrus L. waste are shown in Figure 2. While some of these wastes are now valorized in various ways, such as non-toxic and effective methods, different techniques used for successful extraction might fundamentally improve their valorization and produce greater revenues and high-quality bioactives [29][27].
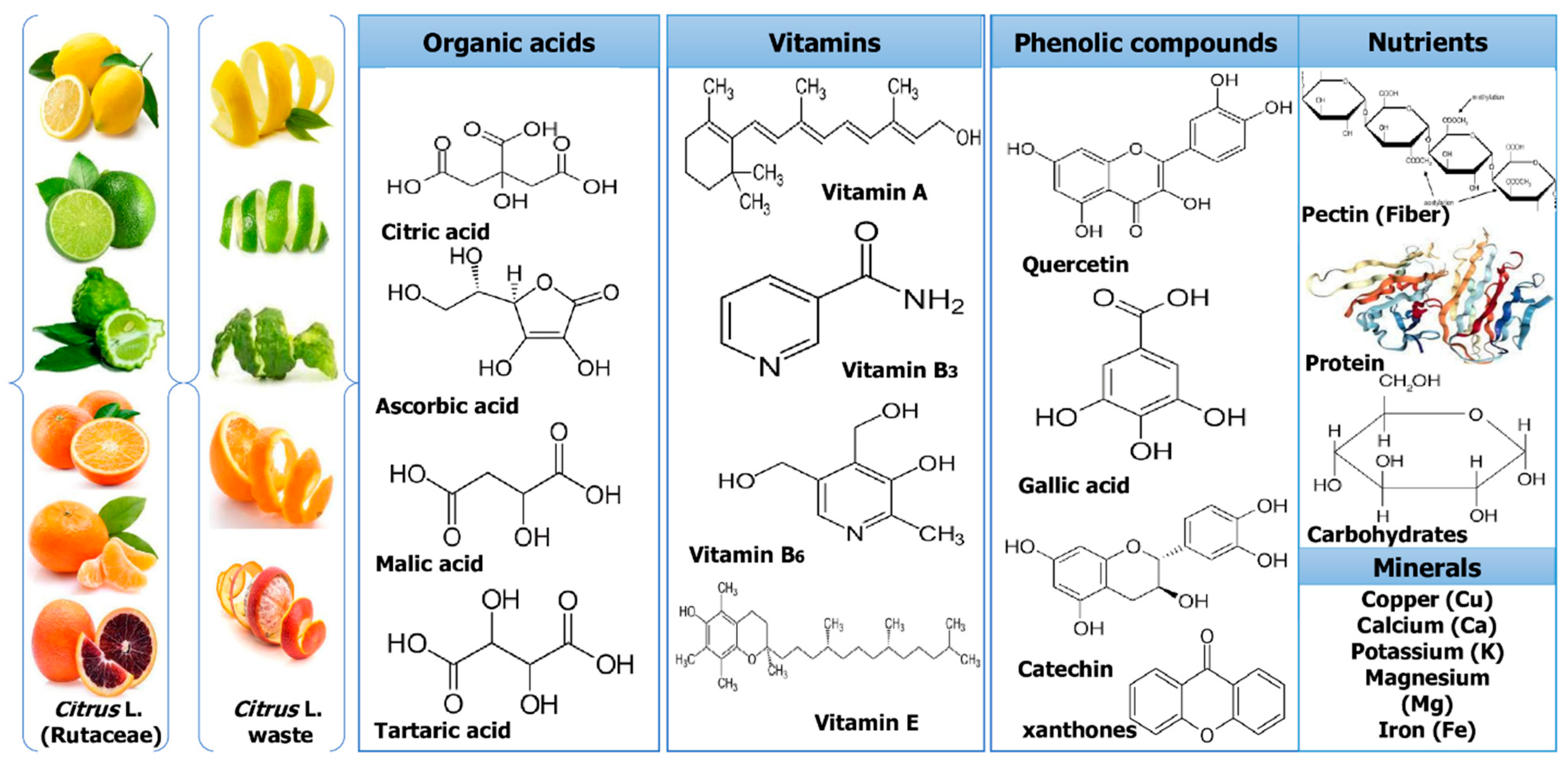
Figure 2. Major phytochemicals of Citrus L. waste.
Citrus by-products contain bioactive compounds with biological activity, including carotenoids, polyphenols, and essential oils (EOs). Carotenoids and polyphenols have numerous health benefits that are related to their antioxidant activity [30][28]. Citrus fruits are frequently processed into turbid juices, and the residue part is considered waste, including juice vesicles, membranes, peels, and seeds. These account for 45 to 60% of the weight discarded [31][29]. Due to the abundance of polyphenols, EOs, flavonoids, dietary fiber, sugars, ascorbic acid, and carotenoids in orange peel, it can be utilized as a source of economically useful, elevated components, in areas such as solid biofuel, bioabsorbents, animal feed, fertilizer, EOs, pectin, ethanol, methane, industrial enzymes, and single-cell protein [32,33,34,35][30][31][32][33].
3.1. Pectin
Ngouémazong et al. [42][34] described pectin as an emulsifier, texturizer, thickener, and stabilizer in food. It is utilized in a variety of applications, including fillings for confectionery and dietary fiber supplements [43][35]. It is derived commercially from the peels of orange, lime, lemon, and grapefruit as a white to pale brown powder [44][36]. The extraction of pectin is among the most efficient and cost-effective methods [45][37]. In a factory setting, citrus peels and rinds are heated to approximately 100 °C and subjected to acidic conditions to extract pectin [46][38]. Alternative extraction methods for pectin extraction have recently been developed, including ultrasonic extraction (USE) [47][39], microwave-assisted extraction (MAE) [48][40], and enzymatic extraction [49][41]. The hydrolysis of lignocellulosic materials has been greatly aided by the application of subcritical water extraction [50][42], in addition to processing any remaining orange peel for its pectin [51][43]. In addition to its technological capabilities in a variety of commodities, pectin can play a role as a dietary fiber and bioactive compound and act as an anti-cancer agent [52][44] (Figure 3).
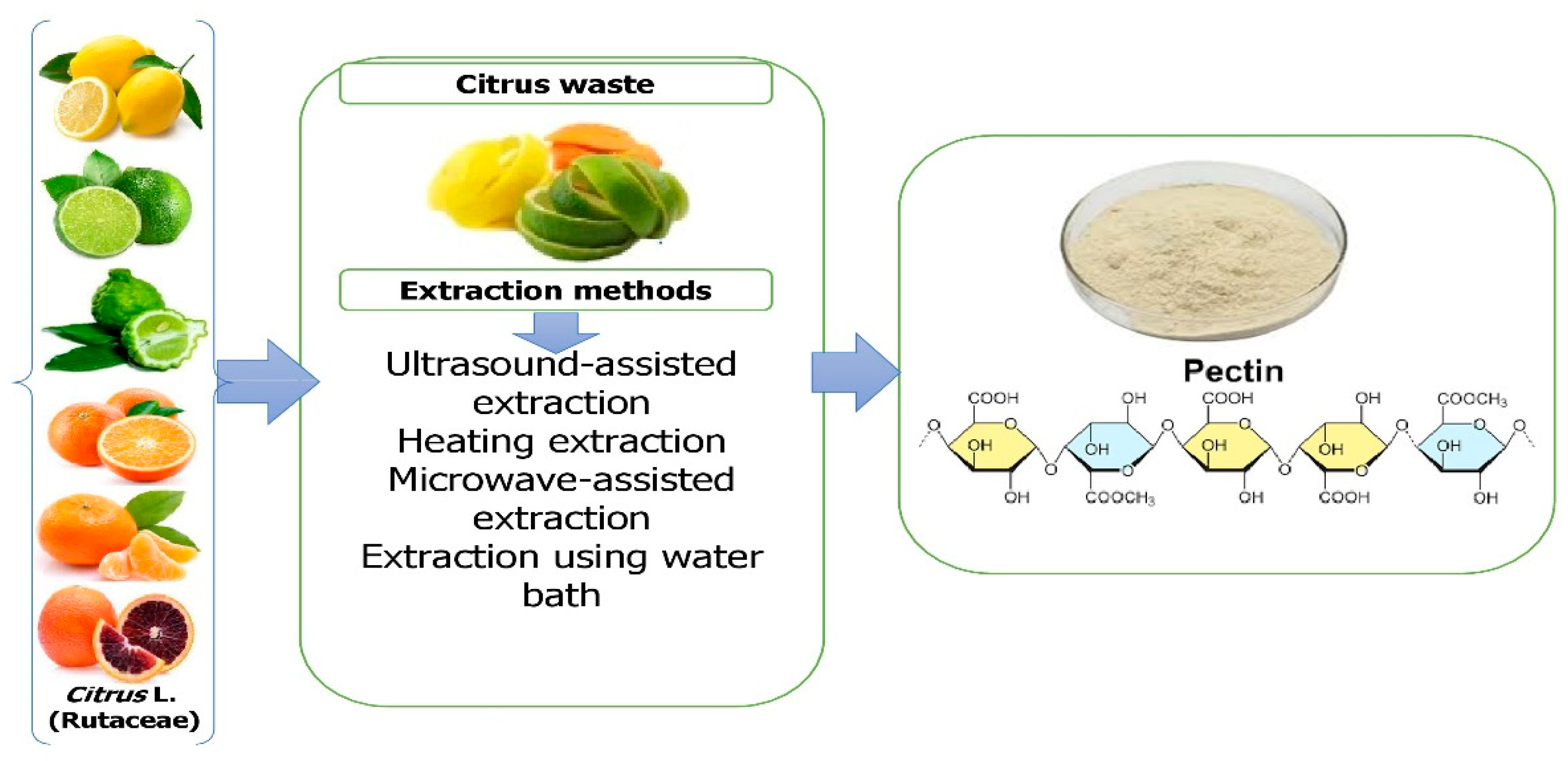
Figure 3. Extraction of pectin from Citrus L. waste.
3.2. Dietary Fiber
Citrus fruits contain both soluble and insoluble fiber that may be separated. Soluble dietary fiber is largely made up of mucus, gum, and pectin; in contrast, hemicellulose, cellulose, and lignin make up the bulk of insoluble dietary fiber [53][45]. Approximately 50–60% of its dry weight can be attributed to cellulose and hemicellulose, which make it a prime source for both substances. Citrus fiber is a biologically active component (BAC) that contains polyphenol-like components and plays a role as a lipid oxidation inhibitor in meat products, prolonging the stability of the meat and enhancing the overall oxidative stability [54][46]. Because of their high water and oil absorption rates, the pulp, seeds, and even the peel of oranges have been utilized as a fat substitute in ice cream [55][47]. Citrus peel is subjected to dietary fiber extraction using subcritical water. Pectin, hemicellulose, and cellulose, together with other dietary fiber sources, were successfully extracted from orange peel through the utilization of a hydrothermal treatment that was carried out at temperatures ranging from 160 to 320 degrees Celsius in a moderate flow extractor. This is an important sequential process for the recovery of useful compounds from citrus fruit waste in a manner that is non-toxic to the environment [56][48].
3.3. Essential Oils (EOs)
Plants create essential oils, which are volatile aromatic compounds and are referred to as EOs. These chemicals have been employed as flavoring agents in food, medicine, and cosmetics since ancient times [57][49]. Citrus species have garnered a great deal of attention due to their abundance of EOs. EOs are highly adaptable and may be used to impart flavor to a large number of products, including beverages, cosmetics, soaps, and household goods [58][50]. EOs have antibacterial, antifungal, and insecticidal properties, as well as antifungal, antibacterial, and insecticidal activity [59][51]. Citrus essential oils were extracted from five citrus species by using hydrodistillation. GC–MS analysis showed different phytochemicals in all five citrus oils, including alkaloids, tannins, sterols, terpenoids, saponins, and limonene [60][52]. A previous study investigated the antioxidant properties, antimicrobial activity, and chemical composition of the essential oil isolated from the aerial parts of Citrus aurantifolia L. The essential oil was extracted using hydrodistillation. Gas chromatography–mass spectrometry (GC–MS) was used to identify and quantify the chemical constituents of the oil. Thirty-three chemical compounds were identified, in which d-limonene was the major constituent. The minor constituents were 3,7-dimethyl-2,6-octadien-1-ol, geraniol, E-citral, Z-citral, and β-ocimene [61][53]. The GC–MS analysis of Citrus aurantium L. (CAEO) identified at least ten compounds, with 2-β pinene, δ-3 carene, and D-limonene as the major compounds [62][54]. The current study was conducted on Citrus medica limonum, in which leaves’ essential oil was extracted by hydrodistillation and the chemical constituents were analyzed by gas chromatography–mass spectroscopy (GC–MS) and Fourier transform infrared (FTIR) spectroscopy. Eleven components were identified in the leaf oil by GC–MS, in which the major constituents were citronellal, limonene, (E)-citral, 1,6-octadien-3-ol, and 3,7-dimethyl [63][55].
3.4. Carotenoids
Carotenoids are also known as carotenes, which are lipid-soluble hydrocarbons, while xanthophylls are their oxygenated derivatives. Carotenes are found in most red and yellow fruits, green leaves, and many roots [64][56]. Carotenoids are plentiful in citrus fruits. Carotenoids (zeaxanthin, lutein, and lycopene), cryptoxanthin, and pro-vitamin are found in the highest concentrations in fruits and vegetables. Carotenoids play an important role in human health. They act as antioxidants and aid in the growth and health of bones. They also exert a positive influence on the immune system, increase cell-to-cell communication, improve eye health, and reduce cancer risks. A previous study demonstrated that carotenoids have many health advantages. The capability of carotenoids to generate vitamin A makes them the only well-established healthier alternative to carotenoids in humans [65][57]. Citrus fruits are a significant source of dietary nutrients due to their high levels of carotenoids. The two primary forms of carotenoids are hydrocarbon carotenoids and oxygenated carotenoids [66][58]. Methanol and diethyl ether were shown to be excellent solvents to extract carotenoids from sour oranges [67][59].
3.5. Polyphenols
The waste of citrus fruits can be used after management in the proper way. The peels offer a superior source of naturally occurring flavonoids and polyphenolics [68][60]. There are six distinct types of flavonoids found in peels concerning their chemical structures, including flavones, flavanols, flavanones, anthocyanidins, isoflavones, and flavonols [69][61]. Flavonoids are phenolic compounds with biological characteristics such as antiallergenic, anti-inflammatory, antiviral, and vasodilation [70][62]. Flavonoids can protect cells from injury by directly scavenging free radicals and preventing their harmful effects [71][63]. To obtain polyphenols from the peels of a variety of citrus fruits, a strategy was employed that combined solid and liquid extraction and was founded on an ethanolic solution in water.
4. Application of Citrus Waste in the Food Industry
Different studies showed that citrus peels contain essential oils that can be used for multiple purposes in food preservation, food safety, and nutraceutical industries. Various research has produced essential oil (EOs)-based thin films, microencapsulation utilizing nanoemulsion coatings, biodegradable polymers, spray applications, and the antibacterial action mechanism of the active chemicals present in EOs [73][64]. Cosmetics, medicines, food formulations, and packaging are a few of the industries that use citrus essential oils (CEOs). CEOs have extensive uses in the areas of food safety, packaging, and storage [74][65]. Figure 4 describes the potential applications of Citrus L.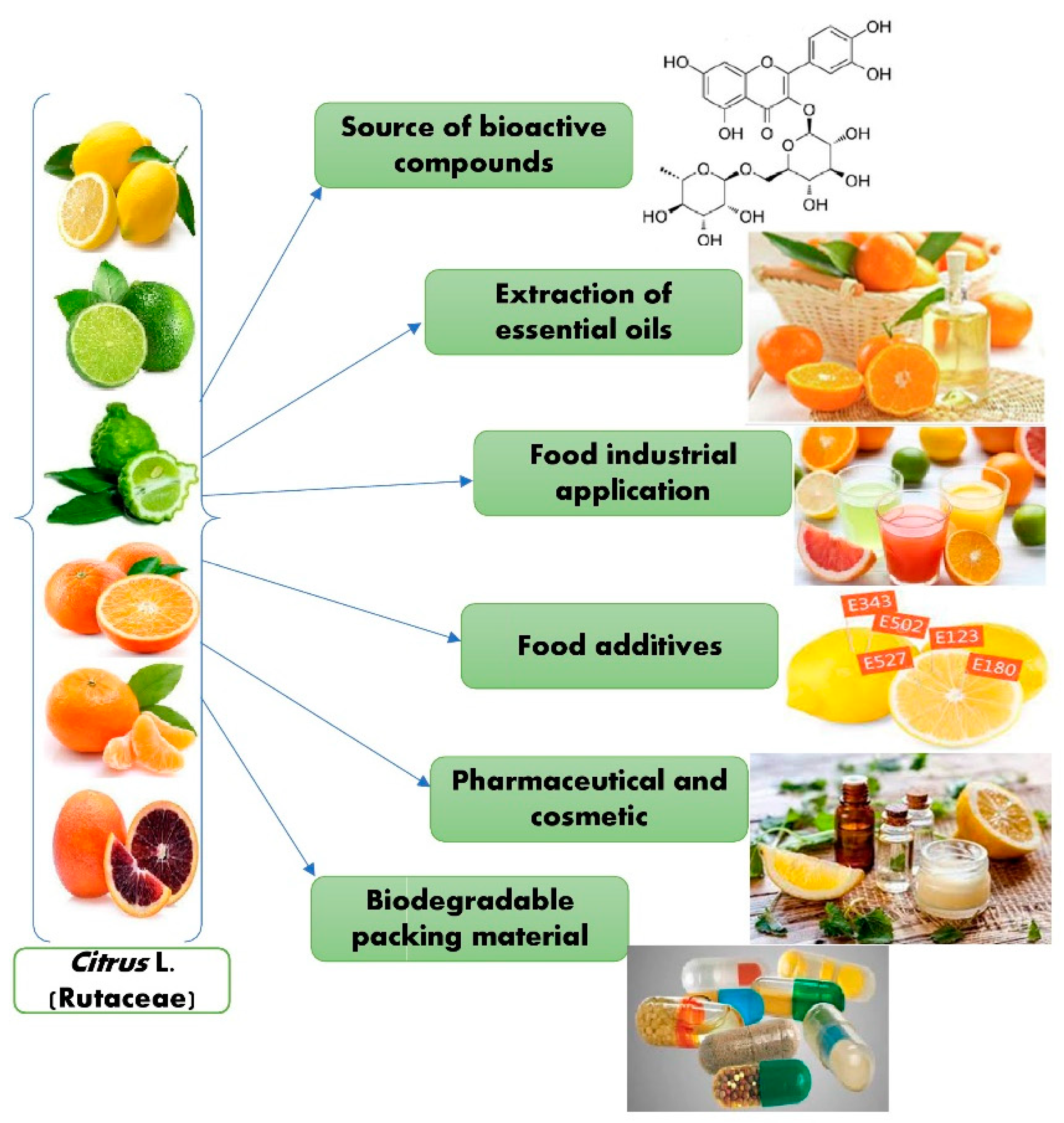
Figure 4. Potential applications of Citrus L.
4.1. Essential Oil-Containing Polysaccharide-Based Edible Films and Coatings
Food industries face a major challenge in the form of waste, which has to be managed and utilized for multiple purposes. Most of the food industries and food scientists use these food wastes as coating materials to protect food products from various environmental factors. Therefore, food coatings and films have gained wide interest due to their excellent palatability and biodegradability, and their ability to maintain food freshness during storage. In the last decade, polysaccharides have been investigated as a viable material for edible films. Applications related to microbiological food safety demonstrated that these polymeric materials have enormous untapped potential. In a study, essential oils (EOs) were mixed with polysaccharide matrices to further enhance the functional qualities of edible films [75][66]. In another research work by Perdones et al. [76][67], cold-stored strawberry was coated with chitosan and lemon essential oil and stored in at 5 °C. Chitosan coatings showed no significant effect on the acidity, pH, and soluble solid content of strawberries throughout the storage period. In the food processing industry, the EOs of citrus lemon peel can be used as a natural antibacterial and antioxidant agent. Lemon peel EOs have antibacterial effectiveness against several food-borne diseases [77][68].4.2. Application of Fiber Concentrate in Bakery Products
Romero-Lopez et al. [78][69] conducted a study on the addition of citrus fiber to muffins. A dietary fiber-rich orange bagasse product (DFROBP) boosted the fiber content of the experimental muffins by 40 and 63% relative to a control muffin. By partially substituting wheat flour with DFROBP, it was possible to create baked goods with high levels of total dietary fiber and a high indigestible fraction, dietary traits that may be advantageous for people with varying nutritional requirements [79][70].4.3. Application of Citrus Pectin in Dairy Drinks
Citrus pectin, a polysaccharide found in plant cell walls, is primarily used in the food processing industry as a stabilizer, thickener, and gelling agent. Pectin can be extracted from the peels of various fruits and their waste; it is used in food industries and is extracted from citrus peels and apple pomace. Pectin is commonly used as a stabilizer agent in acidified milk beverages and yogurts. Acidified milk drinks are very popular due to their natural taste and high nutritional value. Many research works have shown that protein flocculation and whey separation in acidified milk beverages are due to the absence of a stabilizer agent. Furthermore, citrus pectin can be added as a protective colloid to stop this behavior and stabilize milk drinks [80][71].4.4. Utilization of Citrus Waste in the Beverage Industry
Utilizing citrus waste in the food sector is vital due to its bio-functional properties and its potential as a useful approach to improving food quality and customer health. It contributes to the creation of nutrient-rich foods and beverages of superior quality. Citrus waste contains a high level of vitamin C, and it can be used to produce juices and other nutrient-rich beverages [81][72]. Citrus waste also contains essential oils (EOs). These EOs can be used in a variety of beverages, food additives, and pharmaceutical, food, and cosmetic products, because they can provide flavor and impart a sweet aroma (fragrance). Due to the abundance of phenols and carotenoids in citrus manufacturing by-products, these materials can be put to good use as biofertilizers to increase the freshness and longevity of food and drink [82][73].4.5. Applications of Essential Oils (EOs) for Food Safety
For a very long time, EOs have been used in food processing and preservation industries for multiple purposes. Recently, researchers and scientists have focused on antibacterial packaging for food products, edible thin films, effective antibacterial films, nanoemulsions for the preservation of fruits and vegetables, soda/citrus concentrate components, flavoring agents in carbonated colas, soft drinks, and the preservation of meat, fish, and shellfish [73][64]. EOs are more complex than other types of oils because they contain a variety of naturally occurring bioactive chemicals that are frequently utilized in the food industry as superior substitutes. The antioxidant, antibacterial, and antifungal properties of EOs have been investigated in many research works. EOs obtained from tea tree oil, cinnamon oil, sesame oil, clove oil, lemon oil, chia oil, and thyme oil are only a few examples of oils that have significantly increased antioxidant and antibacterial activity, along with increased cereal storage life and enhanced food security [83][74]. The key EO groups, such as terpenes and aromatic volatile compounds, contribute to food safety without compromising quality. Because of their varied actions, including antibacterial and antioxidant capabilities, EOs could replace some chemical preservatives, extending the freshness of foods including cereals and vegetables [74][65].
References
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896.
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Com. Rev. Food Sci. Food Saf. 2012, 11, 530–545.
- Rawat, N.; Kiran, S.P.; Du, D.; Gmitter, F.G., Jr.; Deng, Z. Comprehensive meta-analysis, co-expression, and miRNA nested network analysis identifies gene candidates in citrus against Huanglongbing disease. BMC Plant Biol. 2015, 15, 184.
- Anwar, F.; Naseer, R.; Bhanger, M.; Ashraf, S.; Talpur, F.; Aladeduny, F. Physico-chemical characteristics of citrus seeds and seed oils from Pakistan. J. Am. Oil Chem. Soc. 2008, 85, 321–330.
- Abirami, A.; Nagarani, G.; Siddhuraju, P. The medicinal and nutritional role of underutilized citrus fruit Citrus hystrix (Kaffir lime): A review. Drug Invent. 2014, 6, 1–5.
- Okwu, D.E. Citrus fruits: A rich source of phytochemicals and their roles in human health. Int. J. Chem. Sci. 2008, 6, 451–471.
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68.
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239.
- Ke, Z.; Xu, X.; Nie, C.; Zhou, Z. Citrus flavonoids and human cancers. J. Food Nutr. Res. 2015, 3, 341–351.
- Adenaike, O.; Abakpa, G.O. Antioxidant Compounds and Health Benefits of Citrus Fruits. Eur. J. Nutr. Food Saf. 2021, 13, 65–74.
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Citrus fruits—Morphology, taxonomy, composition, and health benefits. In Fruits Grown in Highland Regions of the Himalayas; Springer: Cham, Switzerland, 2021; pp. 229–244.
- Bursać Kovačević, D.; Gajdoš Kljusurić, J.; Putnik, P.; Vukušić, T.; Herceg, Z.; Dragović-Uzelac, V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chem. 2016, 212, 323–331.
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46.
- Arshad, M.S.; Khalid, W.; Khalid, M.Z.; Maqbool, Z.; Ali, A.; Kousar, S.; Afzal, M.F.; Mukhtar, S.; Rahim, M.A.; Aziz, A.; et al. Sonication Microwave Synergistic Extraction of Bioactive Compounds from Plants, Animals and Others Agro-Industrial Waste Sources; Elsevier Inc.: Amsterdam, The Netherlands, 2022.
- Loesecke, H.W.V. Bureau of Agricultural and Industrial Chemistry; Agricultural Research Administration: Washington, DC, USA, 1952; Volume 44, pp. 476–482.
- Vattem, D.; Shetty, K. Solid-state production of phenolic antioxidants from cranberry pomace by Rhizopus oligosporus. Food Biotechnol. 2002, 16, 189–210.
- Sir Elkhatim, K.A.; Elagib, R.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219.
- Costanzo, G.; Iesce, M.R.; Naviglio, D.; Ciaravolo, M.; Vitale, E.; Arena, C. Comparative studies on different citrus cultivars: A revaluation of waste mandarin components. Antioxidants 2020, 9, 517.
- Jeong, D.; Park, H.; Jang, B.K.; Ju, Y.; Shin, M.H.; Oh, E.J.; Kim, S.R. Recent advances in the biological valorization of citrus peel waste into fuels and chemicals. Bioresour. Technol. 2021, 323, 124603.
- John, I.; Yaragarla, P.; Muthaiah, P.; Ponnusamy, K.; Appusamy, A. Statistical optimization of acid catalyzed steam pretreatment of citrus peel waste for bioethanol production. Resour.-Effic. Technol. 2017, 3, 429–433.
- Choi, I.S.; Lee, Y.G.; Khanal, S.K.; Park, B.J.; Bae, H.J. A low-energy, cost-effective approach to fruit and citrus peel waste processing for bioethanol production. Appl. Energy 2015, 140, 65–74.
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and extraction optimization of Citrus seeds for food and functional food applications. Food Chem. 2021, 355, 129609.
- Ammerman, C.B.; Arrington, L.R. Re-evaluation of citrus pulp as a feed. In Proceedings of the Florida Nutrition Conference, Gainesville, FL, USA, 2 November 1961.
- Rashid, U.; Ibrahimc, M.; Yasin, S.; Yunus, R.; Taufiq-Yap, Y.H.; Gerhard, K. Biodiesel from Citrus reticulata (mandarin orange) seed oil, a potential non-food feedstock. Ind. Crops Prod. 2013, 45, 355–359.
- Wang, L.; Lee, W.W.; Yang, H.W.; Ryu, B.M.; Cui, Y.R.; Lee, S.C.; Lee, T.G.; Jeon, Y.J. Protective Effect of Water Extract of Citrus Pomace against AAPH-Induced Oxidative Stress In Vitro in Vero Cells and In Vivo in Zebrafish. Preventive Nutr. Food Sci. 2018, 23, 301–308.
- Iqbal, A.; Schulz, P.; Rizvi, S.S. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present Insights and future challenges. Food Biosci. 2021, 44, 101384.
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Shpigelman, A. Innovative “green” and novel strategies for the extraction of bioactive added value compounds from citrus wastes—A review. Molecules 2017, 22, 680.
- Levaj, B.; Dragović-Uzelac, V.; Bursać Kovačević, D.; Krasnići, N. Determination of flavonoids in pulp and peel of mandarin fruits. Agric. Conspec. Sci. 2009, 74, 221–225.
- Levaj, B.; Putnik, P.; Linardić, I.; Herceg, Z.; Režek-Jambrak, A.; Kovačević-Bursać, D. Influence of pre-treatment on yield and quality of mandarin juices. In Proceedings of the 6th Central European Congress on Food-CEFood Congress, Novi Sad, Serbia, 2 December 2012.
- Sanders, A. Extraction, Encapsulation, and Microfluidization of Naringin from Grapefruit. Doctoral Dissertation, The Ohio State University, Columbus, OH, USA, 2020.
- Waghmode, M.S.; Gunjal, A.B.; Nawani, N.N.; Patil, N.N. Management of floral waste by conversion to value-added products and their other applications. Waste Bio. Val. 2018, 9, 33–43.
- Santos, C.M.; Dweck, J.; Viotto, R.S.; Rosa, A.H.; de Morais, L.C. Application of orange peel waste in the production of solid biofuels and biosorbents. Bioresour. Technol. 2015, 196, 469–479.
- Ángel Siles López, J.; Li, Q.; Thompson, I.P. Biorefinery of waste orange peel. Crit. Rev. Biotechnol. 2010, 30, 63–69.
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The emulsifying and emulsion-stabilizing properties of pectin: A review. Comp. Rev. Food Sci. Food Saf. 2015, 14, 705–718.
- Kumar, V.; Sinha, A.K.; Makkar, H.P.; De Boeck, G.; Becker, K. Dietary roles of non-starch polysaccharides in human nutrition: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 899–935.
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99.
- Shalini, R.; Gupta, D.K. Utilization of pomace from apple processing industries: A review. J. Food Sci. Technol. 2010, 47, 365–371.
- Koubala, B.B.; Kansci, G.; Mbome, L.I.; Crépeau, M.J.; Thibault, J.F.; Ralet, M.C. Effect of extraction conditions on some physicochemical characteristics of pectins from “Améliorée” and “Mango” mango peels. Food Hydrocoll. 2008, 22, 1345–1351.
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013, 20, 222–231.
- Fishman, M.L.; Cooke, P.H. The structure of high-methoxyl sugar acid gels of citrus pectin is determined by AFM. Carbohydr. Res. 2009, 344, 1792–1797.
- Ptichkina, N.M.; Markina, O.A.; Rumyantseva, G.N. Pectin extraction from pumpkin with the aid of microbial enzymes. Food Hydrocoll. 2008, 22, 192–195.
- Heitz, M.; Carrasco, F.; Rubio, M.; Chauvette, G.; Chornet, E.; Jaulin, L.; Overend, R.P. Generalized correlations for the aqueous liquefaction of lignocellulosic. Can. J. Chem. Eng. 1986, 64, 647–650.
- Carr, A.G.; Mammucari, R.; Foster, N.R. A review of subcritical water as a solvent and its utilization for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17.
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An emerging new bioactive food polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73.
- Khalid, W.; Arshad, M.S.; Jabeen, A.; Anjum, M.F.; Qaisrani, T.B.; Suleria, H.A.R. Fiber-enriched botanicals: A therapeutic tool against certain metabolic ailments. Food Sci. Nutr. 2022, 10, 3203–3218.
- Sáyago-Ayerdi, S.G.; Brenes, A.; Goñi, I. Effect of grape antioxidant dietary fiber on the lipid oxidation of raw and cooked chicken hamburgers. LWT-Food Sci. Technol. 2009, 42, 971–976.
- de Moraes Crizel, T.; Jablonski, A.; de Oliveira Rios, A.; Rech, R.; Flôres, S.H. Dietary fiber from orange byproducts is a potential fat replacer. LWT-Food Sci. Technol. 2013, 53, 9–14.
- Tanaka, M.; Takamizu, A.; Hoshino, M.; Sasaki, M.; Goto, M. Extraction of dietary fiber from Citrus junos peel with subcritical water. Food Bioprod. Process. 2012, 90, 180–186.
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Indus. Crops Prod. 2013, 43, 587–595.
- Lemes, R.S.; Alves, C.C.F.; Estevam, E.B.B.; Santiago, M.B.; Martins, C.H.G.; Dos Santos, T.C.L. Chemical composition and antibacterial activity of essential oils from Citrus Aurantifolia leaves and fruit peel against oral pathogenic Bacteria. An. Acad. Bras. Cienc. 2018, 90, 1285–1292.
- Bayala, B.; Bassole, I.H.; Scifo, R.; Gnoula, C.; Morel, L.; Lobaccaro, J.M.A.; Simpore, J. Anticancer activity of essential oils and their chemical components—A review. Am. J. Cancer Res. 2014, 4, 591.
- Javed, S.; Javaid, A.; Nawaz, S.; Saeed, M.K.; Mahmood, Z.; Siddiqui, S.Z.; Ahmad, R. Phytochemistry, GC-MS analysis, antioxidant and antimicrobial potential of essential oil from five citrus species. J. Agricul. Sci. 2014, 6, 201.
- Al-Aamri, M.S.; Al-Abousi, N.M.; Al-Jabri, S.S.; Alam, T.; Khan, S.A. Chemical composition and in-vitro antioxidant and antimicrobial activity of the essential oil of Citrus aurantifolia L. leaves grown in Eastern Oman. J. Taibah Uni. Med. Sci. 2018, 13, 108–112.
- Nidhi, P.; Rolta, R.; Kumar, V.; Dev, K.; Sourirajan, A. Synergistic potential of Citrus aurantium L. essential oil with antibiotics against Candida albicans. J. Ethnophar. 2020, 262, 113135.
- Onyeyirichi, I.; Ogechi, N.; Oche, O.; Jerry, U.; Gero, M. Evaluation of chemical constituent of Citrus medica limonum leaf essential oil. J. Phar. Scientic. Innov. 2014, 3, 306–309.
- Walia, A.; Gupta, A.K.; Sharma, V. Role of bioactive compounds in human health. Acta Sci. Med. 2019, 3, 25–33.
- Turner, T.; Burri, B.J. Potential nutritional benefits of current citrus consumption. Agriculture 2013, 3, 170–187.
- Dugo, P.; Škeříková, V.; Kumm, T.; Trozzi, A.; Jandera, P.; Mondello, L. Elucidation of carotenoid patterns in citrus products through comprehensive normal-phase× reversed-phase liquid chromatography. Anal. Chem. 2006, 78, 7743–7750.
- Weissenberg, M.; Schaeffler, I.; Menagem, E.; Barzilai, M.; Levy, A. Isocratic non-aqueous reversed-phase high-performance liquid chromatographic separation of capsanthin and capsorubin in red peppers (Capsicum annuum L.), paprika and oleoresin. J. Chromatogr. A 1997, 757, 89–95.
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.S.; Haruenkit, R.; Lojek, A.; Ĉíž, M.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315.
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479.
- Kore, P.S.; Virk, A.; Peste, A. Evaluation of in vitro Anthelmintic potential of Quercetin against pheretima posthuma. J. Pharm. Res. 2018, 12, 974–976.
- Mahmoud, A.M.; Hernandez Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138.
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 611–625.
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55.
- Anis, A.; Pal, K.; Al-Zahrani, S.M. Essential oil-containing polysaccharide-based edible films and coatings for food security applications. Polymers 2021, 13, 575.
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharv. Biol. Technol. 2012, 70, 32–41.
- Moosavy, M.H.; Hassanzadeh, P.; Mohammadzadeh, E.; Mahmoudi, R.; Khatibi, S.A.; Mardani, K. Antioxidant and antimicrobial activities of essential oil of Lemon (Citrus limon) peel in vitro and in a food model. J. Food Qual. Hazards Control. 2017, 4, 42–48.
- Ortiz-Flores, M.; Portilla-Martínez, A.; Cabrera-Pérez, F.; Nájera, N.; Meaney, E.; Villarreal, F.; Ceballos, G. PXR is a target of (-)-epicatechin in skeletal muscle. Heliyon 2020, 6, e05357.
- Romero-Lopez, M.R.; Osorio-Diaz, P.; Bello-Perez, L.A.; Tovar, J.; Bernardino-Nicanor, A. Fiber concentrate from orange (Citrus sinensis L.) bagasse: Characterization and application as a bakery product ingredient. Int. J. Mol. Sci. 2011, 12, 2174–2186.
- Naseri, A.T.; Thibault, J.F.; Ralet-Renard, M.C. Citrus pectin: Structure and application in acid dairy drinks. In Tree and Forestry Science and Biotechnology; Global Science Books: Ikenobe, Japan, 2008.
- Varmie, E.B.; Mamta Thakur, M. Utilization of citrus processing waste: A review. Pharma. Innov. J. 2021, 10, 682–697.
- Okino Delgado, C.H.; Fleuri, L.F. Orange and mango by-products: Agro-industrial waste as a source of bioactive compounds and botanical versus commercial description—A review. Food Rev. Int. 2016, 32, 1–14.
- Khalid, W.; Arshad, M.S.; Aziz, A.; Rahim, M.A.; Qaisrani, T.B.; Afzal, F.; Ali, A.; Ranjha, M.; Khalid, M.Z.; Anjum, F.M. Chia Seeds (Salvia hispanica L.): A Therapeutic Weapon in Metabolic Disorders. Food Sci. Nutr. 2022, 11, 3–16.
More
