Fetal echocardiography is an excellent tool for accurately assessing the anatomy and physiology of most congenital heart defects (CHDs). Knowledge gathered from a thorough initial fetal echocardiogram and serial assessment assists with appropriate perinatal care planning, resulting in improved postnatal outcomes. However, fetal echocardiography alone provides limited information about the status of the pulmonary vasculature, which can be abnormal in certain complex CHDs with obstructed pulmonary venous flow (hypoplastic left heart syndrome with restrictive atrial septum) or excessive pulmonary artery flow (d-transposition of the great arteries, usually with a restrictive ductus arteriosus). Fetuses with these CHDs are at high risk of developing severe hemodynamic instability with the immediate transition from prenatal to postnatal circulatory physiology at the time of birth. Adjunctive use of acute maternal hyperoxygenation (MH) testing in such cases can help determine pulmonary vascular reactivity in prenatal life and better predict the likelihood of postnatal compromise and the need for emergent intervention.
- fetal echocardiography
- maternal hyperoxygenation test
- congenital heart defects
1. Background
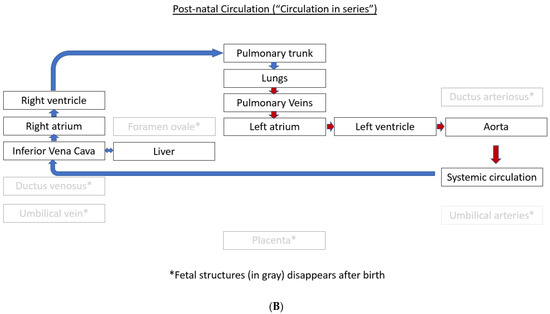
1.1. Fetal Circulation and Transition at the Time of Birth
The normal fetal blood flow pattern (Figure 1A) is characterized by “parallel circulation”, which significantly differs from “in-series” circulation seen after birth (Figure 1B). This parallel fetal circulation is due to three fetal shunts, including ductus venosus, foramen ovale, and ductus arteriosus. The ductus venosus brings the nutrient and oxygen-rich blood from the umbilical vein to the right atrium via the inferior vena cava. The foramen ovale allows for the distribution of nearly half of this systemic venous return to the left side of the fetal heart. The left ventricle (LV) output is mostly distributed to the coronary arteries and the upper part of the fetal body via three branches that originate from the aortic arch. In contrast, most of the output from the right ventricle (RV) passes through the ductus arteriosus to be distributed to the lower part of the fetal body and then back to the placenta via the umbilical arteries. This preferential flow from the RV to the ductus arteriosus is due to the high pulmonary vascular resistance resulting in only a small percentage of the RV output being sent to the branch pulmonary arteries (PAs). Therefore, in fetal circulation, the right and left ventricles are “parallel” to each other, with both ventricles handling a part of the combined cardiac output, and a very small amount going to the lungs.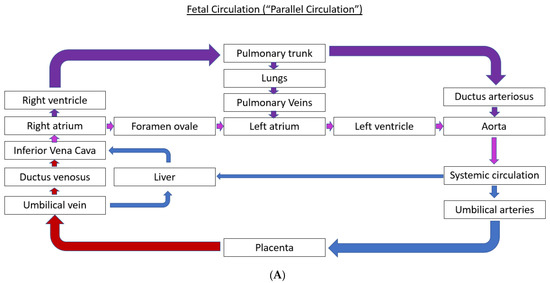
1.2. Historical Perspective on Maternal Hyperoxygenation Testing
Acute MH testing has been studied for over five decades. Studies have focused on evaluating hemodynamic changes to increased circulating oxygen content in the fetal blood, mimicking the postnatal circulatory physiology. Bertolizio et al. [16] and Frangipani et al. [17] published early reports of the effect of MH on amniotic fluid acid–base equilibrium in 1966 and 1969, respectively. However, the cardiac and circulatory changes with MH were not easy to evaluate prior to the availability of fetal echocardiography in the late 1980s. One of the early studies evaluating the cardiac effects of short-term MH reported that abnormal E/A ratio across the mitral and tricuspid valve inflow Doppler patterns seen in growth-restricted fetuses could be improved with MH [18]. Another early study by Soregaroli et al. [19] published in 1993 reported increased peak flow velocities in ductus venosus after MH but no effect on fetal heart rate. This effect of MH on fetal circulation was more obvious in the third trimester compared to early gestational age [20]. A randomized study published in 1997 by Ramner et al. [21] reported that reduction in the pulmonary vascular impedance (measured as pulsatility index in proximal and distal right and left PAs) with MH is significant at 31–36 weeks of gestation but not at 20–26 weeks of gestation. A more detailed evaluation of the fetal echocardiographic findings in the same cohort by Rasanen et al. [22] further characterized this pulmonary vasoreactivity. With acute MH in late gestation, increased pulmonary blood flow was suggested by a reduction in the pulsatility index (PI) in the branch Pas, and a reduction in ductus arteriosus flow was suggested by an increase in the PI of ductus arteriosus. In addition, there was a reduction in flow across the foramen ovale. All of these circulatory changes returned to baseline after MH was discontinued. Again, these changes were observed only in late gestation (31–36 weeks of gestation) fetuses and not in early pregnancy. This development of pulmonary vasoreactivity has been attributed to the smooth muscle development in the fetal PAs during late gestation [23]. Together, these early studies suggested that acute MH could temporarily mimic the postnatal changes in fetal circulation and paved the way to study the utility of MH in guiding postnatal resource preparation in complex CHDs. Additionally, these studies showed that all changes returned to baseline after MH was discontinued, and no untoward side effects were noted in the fetus or the mother, indicating the safety of such testing prenatally.2. Clinical Maternal Hyperoxygenation Protocol
The physiologic change of increased fetal oxygen concentration via MH is achieved by administering oxygen to a mother in late gestation for a short duration. There is no universally accepted MH protocol, but most studies have given 100% humidified oxygen to expectant mothers via a non-rebreather mask for 8 to 15 min (Table 1).| Published Studies Author/Year |
Fetal Characteristics of the Study Cohort | Fetal Cardiac Diagnoses | MH Protocol | Findings |
|---|---|---|---|---|
| Rasanen et al. [22] 1998 |
20 early GA (20–26 weeks) 20 late GA (31–36 weeks) |
|||
| 100% FiO | ||||
| 2 | ||||
| for 10 min via nonrebreather mask at 8 L | ||||
| 10 min recovery | ||||
| ↓ LV strain and strain rate (due to ↑ in cerebral vascular resistance) | ||||
| ↑RV strain and strain rate (due to ↓ in pulmonary vascular resistance) | ↓ Pulmonary artery PI | Most findings did not return to baseline after recovery. |
| Diagnosis | Baseline Fetal Echocardiogram Findings Suggestive of Hemodynamic Instability after Birth | Expected Changes with MH Performed in the Third Trimester Suggestive of Hemodynamic Instability after Birth | Delivery Room Recommendations | |||||
|---|---|---|---|---|---|---|---|---|
| Healthy fetuses | ||||||||
| HLHS and variants with severely restrictive or intact atrial septum | Pulmonary vein Doppler [6]
|
Reduced pulmonary vasoreactivity
| 60% humidified FiO2 for 5 min of MH 5 min of recovery |
↑ PI in DA ↓ Foramen ovale flow -Changes are seen only in late GA and not in early GA fetuses -All changes returned to baseline after 10 min of recovery |
||||
| Szwast et al. [9] 2010 |
30.1 ± 4.5 weeks GA controls 29.6 ± 5.0 |
43 HLHS 27 controls |
100% FiO | |||||
| TAPVR with significant Obstruction | Pulmonary vein Doppler [6]
| 2 | for 10 min via nonrebreather mask at 8 L/min effectively providing 60% inhaled FiO2 5 min of recovery |
-No untoward effects seen with MH |
||||
| Mean gradient in the vertical vein after MH correlates with the severity of TAPVR obstruction seen postnatally | [ | 12] |
|
Zarkowska-Szaniawska et al. [24] 2011 |
late gestation | |||
| D-TGA and variants with a restrictive atrial septum and prenatal ductal constriction | Abnormal foramen ovale [6][32][33][6,32,33]:
| 40 fetuses with cardiomegaly and lung hypoplasia |
Reduced pulmonary vasoreactivity
|
Pulmonary vasoreactivity with MH (>10% reduction in PI in the PA branch) was associated with survival after birth. | ||||
|
|
|
Channing et al. [25] 2015 |
35 ± 3 weeks GA | 12 fetuses with an atrial septal aneurysm affecting LV filling and aortic arch flow | |||
| Severe Ebstein anomaly of the tricuspid valve |
| 100% FiO | 2 for 10 min via nonrebreather mask at 8L/min effectively providing 60% inhaled FiO2 5 min of recovery |
MH altered the atrial septal position (↓ atrial septal excursion), improved LV filling, and normalized aortic flow by increasing pulmonary venous return. -Helpful in differentiating small LV due to atrial septal aneurysm vs. true LV hypoplasia or coarctation of the aorta |
||||
|
|
Enzenberger et al. [11] 2016 |
>26 weeks GA | 22 HLHS | 100% FiO2 for 10 min | ↑ PI in pulmonary veinous Doppler associated with unobstructed atrial septum | ||
|
Pulmonary vasoreactivity with MH > 20% reduction in PI* in the branch PAs and increased cardiac output across the pulmonary valve can predict antegrade flow from the RV to the PA postnatally. The absence of these reassuring findings would be concerning for postnatal hemodynamic instability. |
|
||||||
| Cardiomegaly and lung hypoplasia | Increased cardiothoracic ratio and concerns for significant lung hypoplasia | Poor pulmonary vasoreactivity with MH (<10% reduction in PI in the branch PAs) associated with non-survivors after birth |
|
Schidlow et al. [12] 2018 |
>32 weeks GA | 2 Ebstein 2 TAPVR 4 HLHS 4 d-TGA |
100% FiO2 for 10 min at 10L/min effectively providing 60% inhaled FiO2 15 min recovery |
Reduced pulmonary vasoreactivity (<20% reduction in PI in PA branches) + cardiac anatomic variables based on the lesion assessed |
| Rychik et al. [26] 2018 (Abstract only) |
35.5 ± 2.4 weeks GA | 114 HLHS fetus | 100% FiO2 for 10 min via nonrebreather mask at 8L/min effectively providing 60% inhaled FiO2 5 min of recovery |
No change in Umbilical artery PI (placental resistance unchanged) ↑ cerebral resistance ↓ pulmonary resistance ↑ Ductus arteriosus PI (↑ retrograde flow) No ductal constriction No change in ventricular performance |
||||
| Mardy et al. [13] 2021 | ~34 weeks GA | 27 HLHS fetuses | 100% FiO2 for 10 min via nonrebreather mask at 8 L/min effectively providing 60% inhaled FiO2 at 8L/min | Poor sensitivity with BPA PI Pulmonary Vein F/R VTI < 6.5, 100% Sensitivity and PPV in predicting emergent atrial septoplasty |
||||
| Cox et al. [27] 2022 |
31.0 ± 4.0 weeks for HLHS 27.8 ± 5.1 weeks for controls |
9 HLHS 9 controls |

