Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Jia Xian Law.
Cancer is the second leading contributor to global deaths caused by non-communicable diseases. The cancer cells are known to interact with the surrounding non-cancerous cells, including the immune cells and stromal cells, within the tumor microenvironment (TME) to modulate the tumor progression, metastasis and resistance. Chemotherapy and radiotherapy are the standard treatments for cancers. A new generation of immunotherapy using natural killer (NK) cells, cytotoxic CD8+ T-lymphocytes or macrophages was developed to achieve tumor-specific targeting and circumvent the adverse effects.
- natural killer cells
- extracellular vesicles
- exosomes
- immunotherapy
- cancer
1. Tumor Microenvironment (TME): A Physicochemical Barrier against Immunotherapy
The TME is described as an extremely hostile ecosystem consisting of both physical and chemical components, as illustrated in Figure 31 [69,70][1][2]. Similar to all other cell types, cancerous cells are no exceptions to the innate ability to secrete EVs. In fact, tumor-derived extracellular vesicles (TD-EVs) are expressed in significant quantities, making them a plausible biological marker and progress indicator for cancer patients [71,72,73][3][4][5]. Cancer cells are also more self-sufficient than regular cells since they are capable of producing and responding to their own growth factors [74][6]. As demonstrated by El-Fattah Ibrahim et al. (2019), these tumor-enabling secretions can operate in an autocrine (own cells), paracrine (neighboring cells) and endocrine (distally located cells) manner [75][7]. TD-EVs were known to alter the surrounding microenvironment, making it hostile for immune cells and other healthy tissue, but their utility and mechanism of action were never fully comprehended. However, they do share a resemblance to anti-inflammatory secretions by supporting cell proliferation and angiogenesis, while inhibiting cell apoptosis, maturation or differentiation, and suppressing the recruitment of inflammatory-responsive cells (e.g., NK cells, macrophages, B and T lymphocytes) [76][8]. When healthy cells are replaced or destroyed, the surrounding environment is contaminated by the metabolic waste products, inflammasomes from cell lysis, and cell debris. Active glycolysis in malignant cells consumes the surrounding oxygen and releases acidic products (e.g., pyruvate and hydrogen ions) [77][9]. After depleting the surrounding oxygen, cancer cells will undergo anaerobic glycolysis, which contributes to further acidification via lactic acid production [78][10]. These manifest into the well-known acidic and hypoxic properties of TME, forming a chemical barrier that limits immune cell penetration.
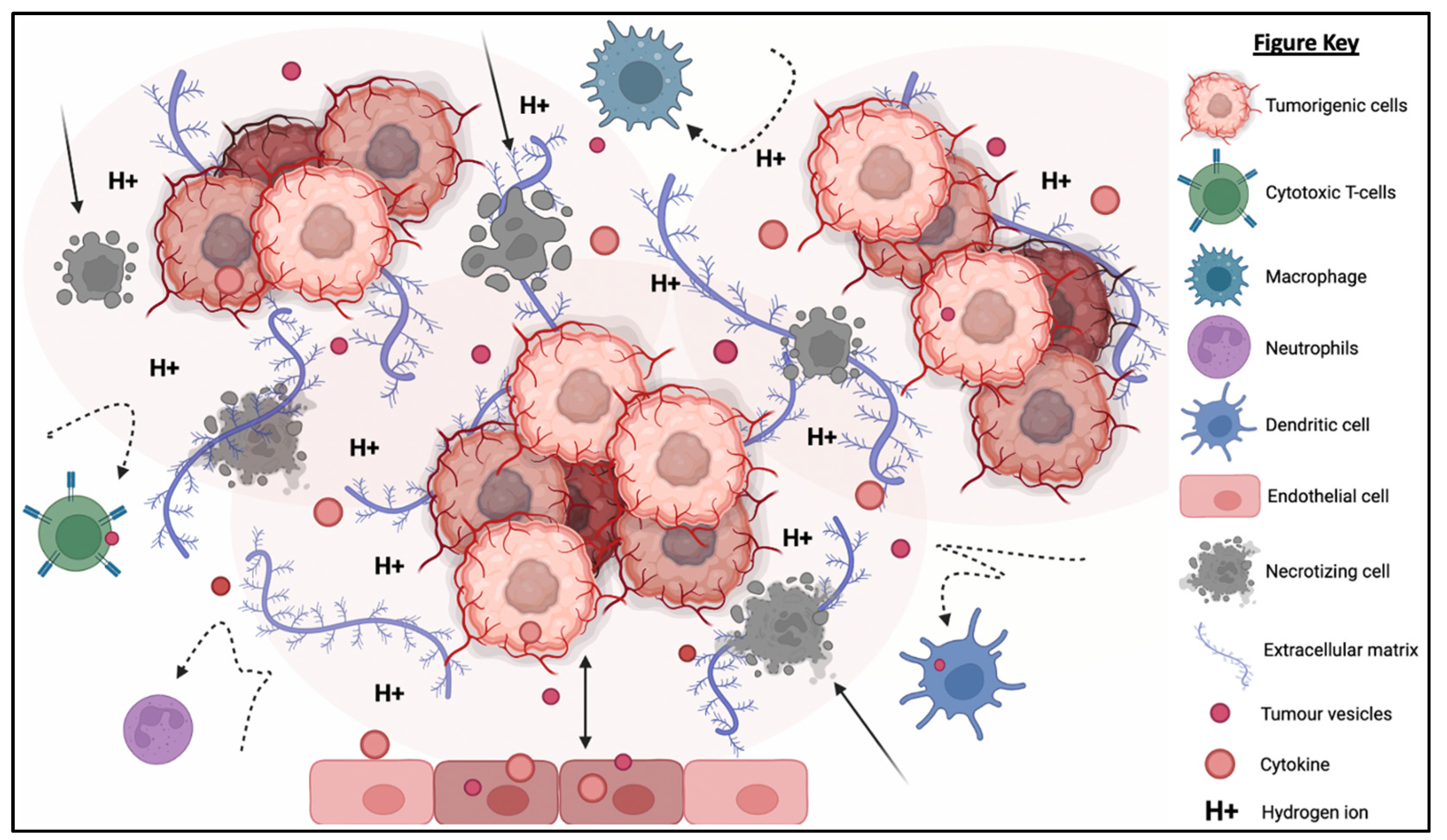
Figure 31. The tumorigenic cells are centered around faulty extracellular matrices, necrotic bodies, and a hypoxic and acidified microenvironment. This tumor microenvironment discourages immunoreactive cells from responding or otherwise entering the tumor to eradicate the tumor cells. At the same time, the hypoxic and acidic environment will induce cell death and exacerbate the inflammation. Moreover, tumor-derived extracellular vesicles will reprogram the host’s cells (e.g., endothelial cells) to support tumor development via increasing the secretion of growth factor, pro-angiogenic and anti-inflammatory cytokines.
What is also seemingly peculiar about TD-EVs is the manipulation of host cells, akin to a virus. TD-EVs are able to influence or reprogram the recipient cells to cooperate with tumors [79][11]. For example, TD-EVs can falsely trigger the anti-inflammatory machinery of the healthy cells to reduce the TME inflammation. On the other hand, death cells in TME will continuously release inflammatory particles. Hence, TME paradoxically houses both anti-inflammatory particles and pro-inflammatory particles. How these opposing factors co-exists and modulate the TME inflammation is yet to be fully understood. In addition, studies have shown that the circulating inflammatory bodies passively recruit immunoreactive cells to the tumor region [80,81,82,83][12][13][14][15]. The immune cells recruited to the TME will be modulated by the TD-EVs to deactivate its tumor cell killing function and/or to maintain its status quo via overstimulation of the inhibition:activation signal ratio [84][16]. At the same time, the aggregation of naïve/resting or inactivated immune cells interlocked by faulty ECMs obstructs any movement and acts as a shield against activated immune cells or medical interventions (e.g., chemotherapy drugs). Thus, the formation of this pseudo-barrier reinforces the physical impenetrability of the tumor, contributing to drug resistance.
TME and TD-EVs are known to work in harmony to disrupt NK cell function, thus, reducing the effectiveness of NK cell therapy. The acidic pH, as well as the immunosuppressive myeloid derived suppressor cells’ (MDSCs) and M2 macrophages’ presence in the TME, suppress the activation of NK cells, thereby compromising their cytotoxicity against a range of tumor cells [85][17]. Similarly, the TD-EVs were found to impair the function of NK cells through the transfer of multiple immunosuppressive factors (e.g., miRNAs and TGF-β) [86,87][18][19]. The existence of physical and chemical barriers in TEM paired with the inhibitory factors from TD-EVs render the NK cells and other immune cells almost entirely incompetent. Cellular release and uptake of EVs are known to increase in acidic environments [88][20]. Additionally, the pH-related stress also increases the EVs’ protein content and surface electrokinetic potential (zeta potential) [89][21]. The EVs with higher surface charges can bind strongly to the cell membrane, therefore increasing its internalization via receptor or non-receptor endocytosis. The higher EV absorption is not restricted to TD-EVs, but applicable to other EVs as well. This implies the possibility of higher absorption of NK-EVs and other immune cell-derived EVs by the cancer cells.
2. NK-EVs Overcome the Chemical and Physical Barriers of TME
NK-EVs inherit the components and functions of NK cells to exert cytotoxicity against tumor cells in vitro and in vivo. NK-EVs not only express tumor-targeting ability but also possess homing and migratory properties via chemotaxis [45,46,47,50,58][22][23][24][25][26]. As depicted in Figure 62, NK-EVs’ framework is significantly more compact (30–200 nm) and they are also more biostable than NK cells [94,95][27][28]. These properties enable them to have improved infiltration and survival in the hostile TME. It has been demonstrated that NK-EVs express NKG2D, FasL and TRAIL just like the parent cells for receptor-mediated apoptosis. Lytic proteins such as PFN and Gzm also exist ubiquitously in NK-EVs to initiate caspase-dependent apoptosis pathway. All these functions show that the simple configuration of NK-EVs does not compromise their capability to eliminate cancer cells through the multiple cytotoxic pathways utilized by its parent cells. Instead, the NK-EVs might be more effective without the interference of the TME and TD-EVs that halt the production of PFN and Gzm in NK cells. It is believed that the anti-cancer potency of NK-EVs is mainly attributed by these killer proteins. However, the concentration and ratio of NK-EVs in relative to TD-EVs in the TME will likely determine if they will be able to exert anti-tumor effects. It is highly possible that the level of inhibitory signals induced by TD-EVs may overwhelm the activating signals of NK-EVs to prevent any cytotoxic action [84][16]. This means that the dose and delivery method of NK-EVs in vivo need to be critically evaluated to ensure their high bioavailability in the tumor in order to exert their cytotoxic function.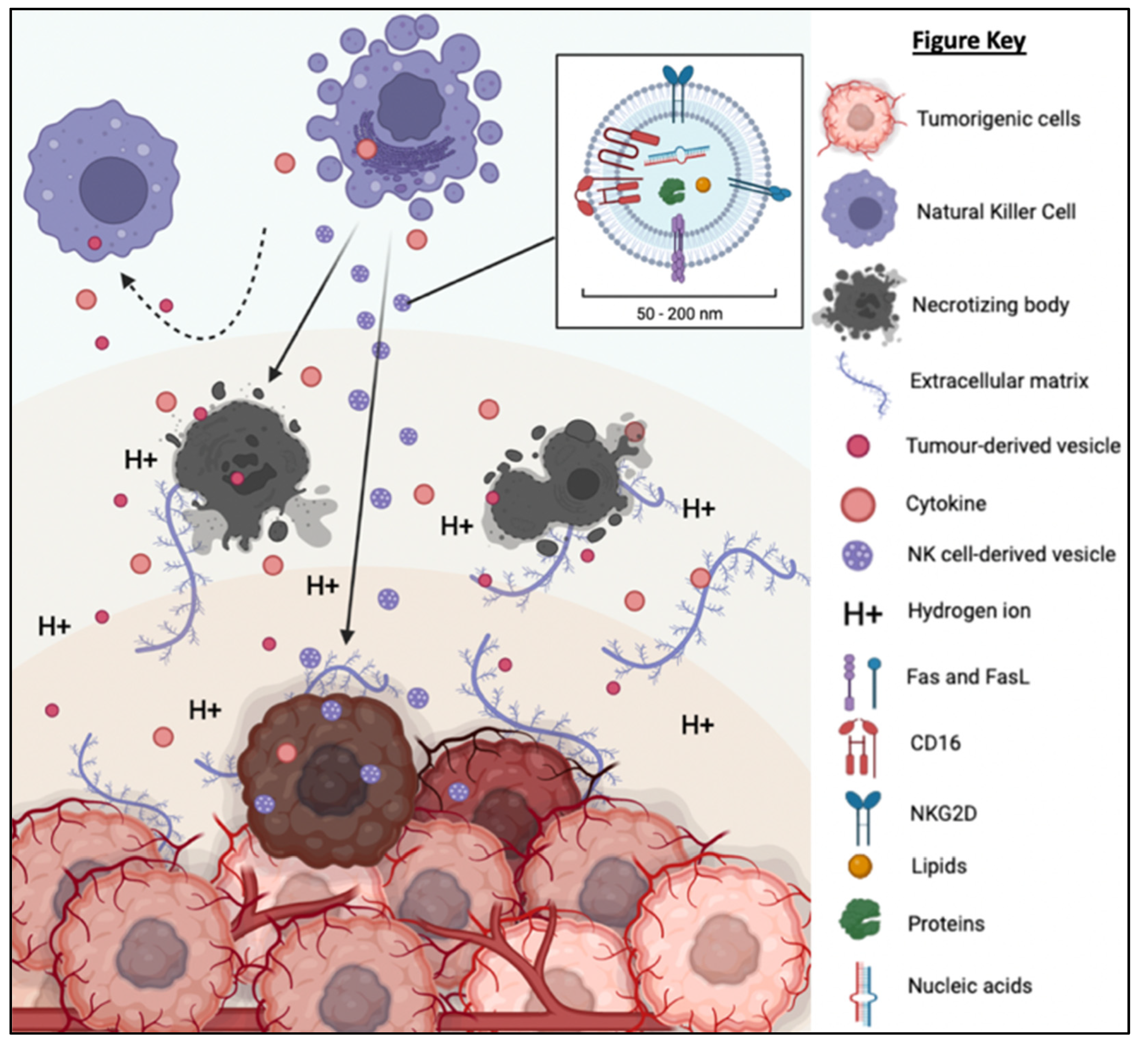
Figure 62. TD-EVs suppress the recruitment and migration as well as reduce the proliferation, survival and cytolytic function of NK cells. On the other hand, NK-EVs secreted by the NK cells are small and diligent enough to overcome the physical and chemical barriers of TME to reach and exert its cytolytic effect on the tumor cells.
3. NK-EVs Are More Clinically Applicable than NK Cells
Results from the in vitro and in vivo experiments demonstrated that NK-EVs are an effective and safe immunotherapy. However, there are several foreseeable issues that need to be addressed in preparing this therapy for future clinical trials and registration. To begin, the functions of NK cells are distinguishable by their cell activation status [24,96,97][29][30][31]. Generally, both naïve and activated NK cells can secrete NK-EVs [98][32]. However, the activated NK cells are preferable for EV isolation due to their high cell number in in vitro culture. It has been reported that the NK-EVs derived from inactivated NK cells have lower amounts of cytotoxic proteins [46][23]. In a separate study, the authors found that EVs secreted by the NK92 cell line contain fewer cytotoxic proteins compared to the EVs secreted by in vitro-expanded NK cells [48][33]. Additionally, Shoae-Hassani et al. (2017) reported that NK cells exposed to neuroblastoma cells produced NK-EVs with higher cytotoxicity towards the tumor cells in vitro and in vivo compared to the NK-EVs derived from naïve NK cells [99][34]. Priming of NK cells with IL-15 was also reported to increase the cytotoxicity of NK-EVs [49][35]. These observations show that NK-EVs are highly heterogeneous depending on the cellular origin, culture environment and physiological status. Therefore, it is important to conduct experiments to the optimize the NK cell culture protocol in order to harvest NK-EVs with potent therapeutic potential. Importantly, the optimization should also consider increasing the yield of NK-EVs in order to reduce the cost. Unlike the stem cell, donor-derived NK cells have variable and limited expansion potential in vitro [100][36]. Thus, efforts have been made to prepare immortalized NK cell lines, such as the NK92-MI from the American Type Culture Collection (ATCC, USA). Cryopreservation is widely used for long-term storage of NK cells. However, the cryopreserved NK cells have relatively poor viability albeit different freezing medium have been tested. Many studies have reported cell viability less than 10% after revival [101][37]. More alarming is that the survived cells ceased proliferating and failed to exert any cytotoxic functions [102][38]. Failure of cell cryopreservation will definitely hamper the clinical translation of NK-EV therapy as it will limit the potential of producing them on a large scale and consistently. At the moment, new donor-derived NK cells are used to prepare the NK-EVs, which leads to high batch-to-batch variation. Furthermore, it will also increase the production cost and lead time. Manual cell culture protocol using tissue culture flask is prone to contamination, technical errors and high batch-to-batch variation [103,104][39][40]. To overcome these limitations, efforts should be made to shift the NK cell expansion to a bioreactor platform which is highly automated, allows more control over the culture environment (e.g., glucose concentration, oxygen concentration and pH) and requires less manpower [105][41]. Usage of a highly efficient bioreactor system might also reduce the cost of production [44][42]. Not least, it is important is to optimize the EV isolation protocol, which should allow quick and reliable isolation of EV subpopulation. Last but not least, long-term storage and off-the-shelf availability are highly desirable for NK-EV therapy. To achieve this, researchers need to device a storage condition that can preserve EV stability over a long period of time. As an acellular product, NK-EVs can be kept chill (4 °C) for immediate use or frozen (−20 °C or −80 °C) up to 12 months with acceptable loss of proteins and RNAs [106,107,108,109,110][43][44][45][46][47]. More importantly, the NK-EVs retain their anti-tumor effect. For easier storage and transportation, NK-EVs could be lyophilized. Even though lyophilization has been widely explored for storage of stem cell-derived EVs, it is still unknown whether lyophilization can be used to preserve the NK-EVs.4. Future Considerations of NK-EVs as a Tool for Immunotherapy
Table 4 1 summarizes the established benefits and challenges of NK cells and NK-EVs for immunotherapy. However, the discussion going forward shall emphasize on improving the EV-based immunotherapy as it has yet to be thoroughly explored but already exhibiting significant advantages compared to the former. One of the main benefits of cell-based and EV-based therapies is that they are minimally invasive medical procedures [120][48]. They are safer alternatives with potentially fewer procedural complications to the high-risk populations such as infants and elderly. In contrast, surgical treatment can lead to many serious complications, including induced hemorrhage, post-operative sepsis and extended rehabilitation [121,122][49][50]. Selection of administration route or delivery technique is critical to ensure the safety and efficacy of a therapy [123][51]. Although IT administration would eliminate any concerns over the homing and migration ability of NK-EVs, the IV administration will be especially useful for regions with poor access or penetrability (e.g., brain) [124][52]. In actuality, a larger dose is typically needed for IV route to compensate for the accumulated loss of drugs due to unwanted distribution at the other tissues as well as metabolism and excretion primary at the liver and kidney [125,126,127][53][54][55]. Nonetheless, the continual IV administration of NK-EVs after the tumor elimination could be favourable to ensure complete clearing of tumor cells to minimize the risk of relapse [128,129][56][57]. To date, it is still unclear on the optimal dosage regime of NK-EVs. More research is needed to determine the ideal frequency, dosage and interval of NK-EV treatment.Table 41.
Summary of pros and cons of NK cell vs. NK-EV for immunotherapy.
| Pros | Cons | |
|---|---|---|
| NK CELL |
|
|
| NK-EV |
|
|
References
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Mazeedi, M.A.M.A.; Almazyadi, H.A.M.; Kallmeyer, K.; Dandara, C.; Pepper, M.S.; et al. The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer. Int. J. Mol. Sci. 2017, 18, 1586.
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925.
- Xue, H.; Lu, B.; Lai, M. The cancer secretome: A reservoir of biomarkers. J. Transl. Med. 2008, 6, 52.
- Lin, J.; Ma, L.; Zhang, D.; Gao, J.; Jin, Y.; Han, Z.; Lin, D. Tumour biomarkers—Tracing the molecular function and clinical implication. Cell Prolif. 2019, 52, e12589.
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142.
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036.
- El-Fattah Ibrahim, S.A.; Abudu, A.; Johnson, E.; Aftab, N.; Conrad, S.; Fluck, M. Correction: The role of AP-1 in self-sufficient proliferation and migration of cancer cells and its potential impact on an autocrine/paracrine loop. Oncotarget 2019, 10, 799, Erratum for: Oncotarget 2018, 9, 34259–34278.
- Qiao, F.; Pan, P.; Yan, J.; Sun, J.; Zong, Y.; Wu, Z.; Lu, X.; Chen, N.; Mi, R.; Ma, Y.; et al. Role of tumor-derived extracellular vesicles in cancer progression and their clinical applications (Review). Int. J. Oncol. 2019, 54, 1525–1533.
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126.
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89.
- Ribeiro Franco, P.I.; Rodrigues, A.P.; de Menezes, L.B.; Pacheco Miguel, M. Tumor microenvironment components: Allies of cancer progression. Pathol. Res. Pract. 2020, 216, 152729.
- Li, P.; Lu, M.; Shi, J.; Hua, L.; Gong, Z.; Li, Q.; Shultz, L.D.; Ren, G. Dual roles of neutrophils in metastatic colonization are governed by the host NK cell status. Nat. Commun. 2020, 11, 4387.
- Nowak, M.; Klink, M. The Role of Tumor-Associated Macrophages in the Progression and Chemoresistance of Ovarian Cancer. Cells 2020, 9, 1299.
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 2020, 17, 807–821.
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946.
- Hodge, G.; Barnawi, J.; Jurisevic, C.; Moffat, D.; Holmes, M.; Reynolds, P.N.; Jersmann, H.; Hodge, S. Lung cancer is associated with decreased expression of perforin, granzyme B and interferon (IFN)-γ by infiltrating lung tissue T cells, natural killer (NK) T-like and NK cells. Clin. Exp. Immunol. 2014, 178, 79–85.
- Cao, L.; Huang, T.; Chen, X.; Li, W.; Yang, X.; Zhang, W.; Li, M.; Gao, R. Uncovering the interplay between pH receptors and immune cells: Potential drug targets (Review). Oncol. Rep. 2021, 46, 228.
- Zhao, J.; Schlößer, H.A.; Wang, Z.; Qin, J.; Li, J.; Popp, F.; Popp, M.C.; Alakus, H.; Chon, S.H.; Hansen, H.P.; et al. Tumor-Derived Extracellular Vesicles Inhibit Natural Killer Cell Function in Pancreatic Cancer. Cancers 2019, 11, 874.
- Li, Q.; Cai, S.; Li, M.; Salma, K.I.; Zhou, X.; Han, F.; Chen, J.; Huyan, T. Tumor-Derived Extracellular Vesicles: Their Role in Immune Cells and Immunotherapy. Int. J. Nanomed. 2021, 16, 5395–5409.
- Nakase, I.; Ueno, N.; Matsuzawa, M.; Noguchi, K.; Hirano, M.; Omura, M.; Takenaka, T.; Sugiyama, A.; Bailey Kobayashi, N.; Hashimoto, T.; et al. Environmental pH stress influences cellular secretion and uptake of extracellular vesicles. FEBS Open Biol. 2021, 11, 753–767.
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264.
- Zhu, L.; Gangadaran, P.; Kalimuthu, S.; Oh, J.M.; Baek, S.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Novel alternatives to extracellular vesicle-based immunotherapy—Exosome mimetics derived from natural killer cells. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S166–S179.
- Neviani, P.; Wise, P.M.; Murtadha, M.; Liu, C.W.; Wu, C.H.; Jong, A.Y.; Seeger, R.C.; Fabbri, M. Natural Killer-Derived Exosomal miR-186 Inhibits Neuroblastoma Growth and Immune Escape Mechanisms. Cancer Res. 2019, 79, 1151–1164.
- Wang, G.; Hu, W.; Chen, H.; Shou, X.; Ye, T.; Xu, Y. Cocktail Strategy Based on NK Cell-Derived Exosomes and Their Biomimetic Nanoparticles for Dual Tumor Therapy. Cancers 2019, 11, 1560.
- Choi, J.W.; Lim, S.; Kang, J.H.; Hwang, S.H.; Hwang, K.C.; Kim, S.W.; Lee, S. Proteome Analysis of Human Natural Killer Cell Derived Extracellular Vesicles for Identification of Anticancer Effectors. Molecules 2020, 25, 5216.
- Kim, H.Y.; Min, H.K.; Song, H.W.; Yoo, A.; Lee, S.; Kim, K.P.; Park, J.O.; Choi, Y.H.; Choi, E. Delivery of human natural killer cell-derived exosomes for liver cancer therapy: An in vivo study in subcutaneous and orthotopic animal models. Drug Deliv. 2022, 29, 2897–2911.
- van Dommelen, S.M.; Vader, P.; Lakhal, S.; Kooijmans, S.A.; van Solinge, W.W.; Wood, M.J.; Schiffelers, R.M. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J. Control Release 2012, 161, 635–644.
- Kao, C.Y.; Papoutsakis, E.T. Extracellular vesicles: Exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60, 89–98.
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124.
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006, 214, 73–91.
- Hood, S.P.; Foulds, G.A.; Imrie, H.; Reeder, S.; McArdle, S.E.B.; Khan, M.; Pockley, A.G. Phenotype and Function of Activated Natural Killer Cells From Patients With Prostate Cancer: Patient-Dependent Responses to Priming and IL-2 Activation. Front. Immunol. 2019, 9, 3169.
- Lugini, L.; Cecchetti, S.; Huber, V.; Luciani, F.; Macchia, G.; Spadaro, F.; Paris, L.; Abalsamo, L.; Colone, M.; Molinari, A.; et al. Immune surveillance properties of human NK cell-derived exosomes. J. Immunol. 2012, 189, 2833–2842.
- Wu, C.H.; Li, J.; Li, L.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C.; Jong, A.Y. Extracellular vesicles derived from natural killer cells use multiple cytotoxic proteins and killing mechanisms to target cancer cells. J. Extracell Vesicles 2019, 8, 1588538.
- Shoae-Hassani, A.; Hamidieh, A.A.; Behfar, M.; Mohseni, R.; Mortazavi-Tabatabaei, S.A.; Asgharzadeh, S. NK Cell-derived Exosomes From NK Cells Previously Exposed to Neuroblastoma Cells Augment the Antitumor Activity of Cytokine-activated NK Cells. J. Immunother. 2017, 40, 265–276.
- Zhu, L.; Kalimuthu, S.; Oh, J.M.; Gangadaran, P.; Baek, S.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Enhancement of antitumor potency of extracellular vesicles derived from natural killer cells by IL-15 priming. Biomaterials 2019, 190–191, 38–50.
- Fehniger, T.A.; Bluman, E.M.; Porter, M.M.; Mrózek, E.; Cooper, M.A.; VanDeusen, J.B.; Frankel, S.R.; Stock, W.; Caligiuri, M.A. Potential mechanisms of human natural killer cell expansion in vivo during low-dose IL-2 therapy. J. Clin. Invest. 2000, 106, 117–124.
- Ishikawa, T.; Okayama, T.; Sakamoto, N.; Ideno, M.; Oka, K.; Enoki, T.; Mineno, J.; Yoshida, N.; Katada, K.; Kamada, K.; et al. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with IgG1 antibody in patients with gastric or colorectal cancer. Int. J. Cancer 2018, 142, 2599–2609.
- Sanchez-Martinez, D.; Allende-Vega, N.; Orecchioni, S.; Talarico, G.; Cornillon, A.; Vo, D.N.; Rene, C.; Lu, Z.Y.; Krzywinska, E.; Anel, A.; et al. Expansion of allogeneic NK cells with efficient antibody-dependent cell cytotoxicity against multiple tumors. Theranostics 2018, 8, 3856–3869.
- Manuel, J. Low cost tissue culture technology for the regeneration of some economically important plants for developing countries. Int. J. Agric. Environ. Biotechnol. 2013, 6, 703–711.
- Daniszewski, M.; Crombie, D.E.; Henderson, R.; Liang, H.H.; Wong, R.C.B.; Hewitt, A.W.; Pébay, A. Automated Cell Culture Systems and Their Applications to Human Pluripotent Stem Cell Studies. SLAS Technol. 2018, 23, 315–325.
- Hassan, M.N.F.B.; Yazid, M.D.; Yunus, M.H.M.; Chowdhury, S.R.; Lokanathan, Y.; Idrus, R.B.H.; Ng, A.M.H.; Law, J.X. Large-Scale Expansion of Human Mesenchymal Stem Cells. Stem Cells Int. 2020, 2020, 9529465.
- Jong, A.Y.; Wu, C.H.; Li, J.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J. Extracell Vesicles 2017, 6, 1294368.
- Wu, J.Y.; Li, Y.J.; Hu, X.B.; Huang, S.; Xiang, D.X. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: A comparative evaluation of storage conditions. Drug Deliv. 2021, 28, 162–170.
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The impact of storage on extracellular vesicles: A systematic study. J. Extracell Vesicles 2022, 11, e12162.
- Sivanantham, A.; Jin, Y. Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos. Life 2022, 12, 697.
- Palay, S.L.; Palade, G.E. The fine structure of neurons. J. Biophys. Biochem. Cytol. 1955, 1, 69–88.
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538.
- Witwer, K.W.; Théry, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell Vesicles 2019, 8, 1648167.
- Ashammakhi, N.; Ahadian, S.; Darabi, M.A.; El Tahchi, M.; Lee, J.; Suthiwanich, K.; Sheikhi, A.; Dokmeci, M.R.; Oklu, R.; Khademhosseini, A. Minimally Invasive and Regenerative Therapeutics. Adv. Mater. 2019, 31, e1804041.
- Pinto, A.; Faiz, O.; Davis, R.; Almoudaris, A.; Vincent, C. Surgical complications and their impact on patients’ psychosocial well-being: A systematic review and meta-analysis. BMJ Open 2016, 6, e007224.
- Cheng, H.; Clymer, J.W.; Po-Han Chen, B.; Sadeghirad, B.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged operative duration is associated with complications: A systematic review and meta-analysis. J. Surg. Res. 2018, 229, 134–144.
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.; Ramakrishna, S. Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Med. Drug Discov. 2022, 15, 100134.
- Jin, J.F.; Zhu, L.L.; Chen, M.; Xu, H.M.; Wang, H.F.; Feng, X.Q.; Zhu, X.P.; Zhou, Q. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer. Adherence 2015, 9, 923–942.
- Lam, W.J.; Bhowmick, T.; Gross, A.; Vanschooneveld, T.C.; Weinstein, M.P. Using higher doses to compensate for tubing residuals in extended-infusion piperacillin-tazobactam. Ann. Pharmacother. 2013, 47, 886–891.
- Bolla, B.; Buxani, Y.; Wong, R.; Jones, L.; Dube, M. Understanding IV antimicrobial drug losses: The importance of flushing infusion administration sets. JAC Antimicrob. Resist. 2020, 2, dlaa061.
- Gao, X.L.; Zhang, M.; Tang, Y.L.; Liang, X.H. Cancer cell dormancy: Mechanisms and implications of cancer recurrence and metastasis. OncoTarget. Ther. 2017, 10, 5219–5228.
- Gomis, R.R.; Gawrzak, S. Tumor cell dormancy. Mol. Oncol. 2017, 11, 62–78.
- Becker, P.S.; Suck, G.; Nowakowska, P.; Ullrich, E.; Seifried, E.; Bader, P.; Tonn, T.; Seidl, C. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol. Immunother. 2016, 65, 477–484.
- Bt Hj Idrus, R.; Abas, A.; Ab Rahim, F.; Saim, A.B. Clinical Translation of Cell Therapy, Tissue Engineering, and Regenerative Medicine Product in Malaysia and Its Regulatory Policy. Tissue Eng. Part A 2015, 21, 2812–2816.
More
