You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Khristine Kaith Sison Lloren and Version 2 by Sirius Huang.
Proteases are the group of enzymes that carry out proteolysis in all forms of life and play an essential role in cell survival. By acting on specific functional proteins, proteases affect the transcriptional and post-translational pathways in a cell. Lon, FtsH, HslVU and the Clp family are among the ATP-dependent proteases responsible for intracellular proteolysis in bacteria. Also, in bacteria, Lon protease acts as a global regulator and governs an array of important functions such as DNA replication and repair, virulence factors, stress response and biofilm formation.
- Lon protease
- bacteria
- virulence
- stress response
- pathogenesis
1. Introduction
A dedicated group of proteases carries out intracellular proteolysis, essential for cell survival. The proteases play a pivotal role in maintaining cellular protein homeostasis by removing damaged, non-functional and short-lived proteins, especially those under stress conditions that threaten the proteome. By regulating the amount of specific functional proteins, the proteases participate in transcriptional and post-translational pathways. In bacteria, intracellular proteolysis is carried out by ATP-dependent proteases that belong to the large AAA+ family proteins (ATPase associated with various cellular activities), such as Lon, FtsH, HslVU and the Clp family (ClpAP and ClpXP) [1][2][1,2]. Among them, FtsH is the only essential and membrane-anchored protease [3], and Lon is present in all of the kingdoms of life. Lon dominates proteolysis in the cytosols of bacteria, the plasma membranes of archaea and the mitochondria of eukaryotes [4]. In Escherichia coli, more than half of the intracellular proteolysis is carried out by Lon protease [5]. The bacterial Lon protease (LonA) consists of three major domains: the N-terminal domain (NTD) that oligomerizes and recognizes substrates, the hexameric AAA+ (A) domain with unfolding activity, i.e., ATP binding and hydrolysis, and the C-terminal serine protease (P) domain, which hydrolyses substrates [6][7][6,7]. In the absence of a substrate, the enzyme’s hexameric ring adopts an open conformation. On the other hand, in the closed substrate-engaged form, sequential ATP hydrolysis in the adjoining subunits around the ring drives the translocation of unfolded substrates from the A domain towards the P domain via binding to a staircase of aromatic residues [8][9][8,9]. This mechanism is most likely to be conserved in all Lon proteases and is related to the rotary treadmilling mechanism of other AAA+ translocases [10]. Cryo-EM structure studies of the human counterpart called LONP1 protease, which is found in the mitochondria, revealed the structural conservation between human and bacterial Lon protease, which is suggestive of similar mechanisms of action [11][12][11,12]. Because of their important cellular functions, human Lon proteases are considered to be promising therapeutic targets [13], and their corresponding bacterial homologs are considered to be potential antimicrobial drug targets [14]. Hence, advancing the knowledge of the substrate pools of specific proteases and the mechanisms underlying substrate selection is vital.
2. Structure of Bacterial Lon Protease
Lon proteases assemble into barrel-shaped homo-hexamers, with the proteolytic active sites sequestered in an internal chamber, and they are largely inaccessible to folded proteins; this architecture serves to prevent the degradation of non-substrate proteins [9]. A single monomer of Lon contains an ATPase AAA+ molecular chaperon and a proteolytic domain with a serine-lysine catalytic dyad. Additionally, the bacterial Lon proteases (LonA) also contain an extra two-domain N-terminal region, with all of the domains being part of a single polypeptide chain. The N-domain is essential for the oligomerization of LonA proteases and is involved in the binding of protein substrates and their hydrolysis. In Escherichia coli, the enzyme assembles into a hexamer with an internal degradation chamber accessible via an axial pore in the AAA+ ring [15][36]. The Lon hexameric ring recognizes a substrate, unfolds the protein if it is necessary by ATP-dependent reactions mediated by the AAA+ pore and translocates the denatured polypeptide through a central axial pore and into the proteolytic chamber for degradation [15][16][36,37] (Figure 12).
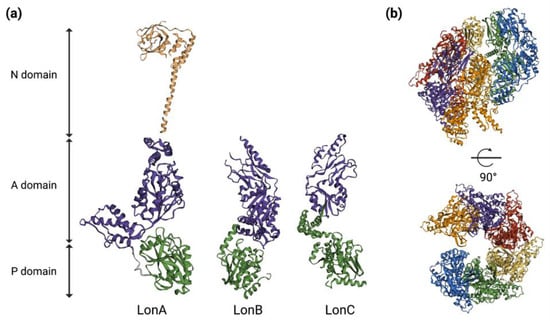
Figure 12. Three-dimensional structures of Lon protease. (a) Representative 3D models of single protomers of LonA (PDB ID: 6u5z & 3ljc) of E. coli, LonB of Thermococcus onnurineus (PDB ID: 3k1j) and LonC of Meiothermus taiwanensis (PDB ID: 4fw9). (b) Cryo-EM structure of LonA in E. coli (PDB ID: 6u5z) without a substrate. Six different colors represent six chains that form the hexamer. The 3D structures and PDB IDs were adapted from Wlodawer et. al., 2022 [17][38]. P = Protease; A = ATPase; N = N-terminal.
Apart from the well-known hexameric form of Lon protease, larger oligomeric forms of the enzyme are reported in Escherichia coli, Bacillus subtilis and Mycobacterium smegmatis [15][18][19][36,39,40]. In Escherichia coli, Lon forms dodecamers that equilibrate with hexamers at a physiological concentration. Here, the N-domain interactions between the Lon hexamers lead to the formation of a Lon dodecamer with an altered, yet unique, substrate with degradation properties. For the protein substrates to gain access to the degradation machinery in the dodecamer, they need to pass through the equatorial portals. By doing so, the dodecamers prevent proteolysis of the large, but not small protein substrates. This selective protein degradation helps to maintain the intracellular repertoire of the Lon substrate protein [15][36]. An unexpected dimeric interaction between the N-terminal domains and the Lon monomers is thought to initiate a possible dodecamer formation in Bacillus subtilis. Here, a domain swap arrangement in the N-terminal domain dimers may facilitate the head-to-head dimerization of hexamers, which might help in the formation of a dodecamer [18][39]. Ion-dependent oligomerization is essential for Lon protease activity in Mycobacterium smegmatis. At low Mg2+ concentrations, the cellular conditions favor the formation of lower oligomeric forms of Lon, whereas at higher Mg2+ concentrations, the enzyme exists in higher-order oligomers [20][41].
3. Substrate Specificity of Bacterial Lon Protease
Even though the exact role of the three Lon domains in substrate selection is unknown, the N-terminal domain of the Escherichia coli Lon is essential for substrate recognition and binding, as shown by the direct binding between Lon and sul20 peptide [7][21][7,42]. Concomitantly, the literature shows that an E240K mutation in the N-terminal domain alters the degradation of substrates, and a truncation of the N-terminal domain completely impairs the Escherichia coli Lon proteolytic activity towards β-casein [22][43]. The Lon proteases are renowned for their ability to degrade misfolded and faulty proteins, which is an observation that has been substantiated by studies carried out in Escherichia coli. Yet, a few proteins undergo Lon-mediated proteolysis even in their native forms, namely SulA [23][44], SoxS [24][45], HupB [25][46], IbpA [26][27], RcsA [27][47] and RpsB [28][48]. Researchers lack elucidations that show how protein substrates are earmarked for degradation by Lon protease. Certain sequence features on the known substrates seem to contribute to their degradation by Lon. In this regard, Lon is similar to other AAA+ proteases that recognize specific peptide signals in substrates called degradation tags or degrons [29][49]. Attaching a degradation tag to a stably folded protein leads to its lysis by Lon [30][50]. In Escherichia coli, a degron SsrA tag (AANDENYALAA) marks the polypeptides for targeted proteolysis by Lon [31][51]. Additionally, the Lon-mediated degradation of certain substrates requires adaptors. This is exemplified by the observation that Bacillus subtilis SmiA facilitates the degradation of SwrA by Lon [32][26]. Furthermore, studies of Yersinia pestis have shown that HspQ acts as a Lon specificity-enhancing factor and enhances the Lon-mediated degradation of a select set of substrates including YmoA, RsuA, Y0390 and Fur [33][52]. Karlowicz et al. reported the degradation of certain DNA-binding proteins, namely TrfA and RepE, which require the interaction of Lon protease with DNA [34][53]. The important bacterial proteins that are substrates of Lon protease are stated in Table 1. Another distinct feature of Lon protease is its organism-specific substrate specificity. A recent study showed that the human Lon protease failed to degrade Francisella tularensis and Escherichia coli Lon substrates. Furthermore, a domain swap between the bacterial and human Lon proteases showed that substrate recognition and biochemical cleavage require there to be an intrinsic match of three domains in the Lon proteases [7]. It is not clear if processive peptide bond hydrolysis without substrate dissociation is necessary for Lon-mediated degradation. The studies across species have shown that Lon proteases degrade the substrate and generate peptide products consisting of ~5–30 amino acids [35][36][54,55].
Table 1.
Important substrates of bacterial Lon protease.
| Bacteria | Substrate(s) | Function(s) |
|---|---|---|
| Escherichia coli Lon | HupB (DNA-binding protein HU-beta) | Histone-like DNA-binding protein that wraps around the DNA to stabilize it and prevent its denaturation under extreme environmental conditions [37][56]. |
| IbpA (Small heat shock protein) | Together with IbpB, it protects the aggregated proteins from irreversible denaturation and extensive proteolysis during heat shock and oxidative stress [38][57]. | |
| RcsA (Transcriptional regulatory protein) | Component of the Rcs signaling system, which controls the transcription of an array of genes. RcsA binds RcsB to the RcsAB box to regulate gene expression [39][58]. | |
| RpsB (30S ribosomal protein S2) | Required for ribosomal protein S1 to bind to the 30S subunit [40][59]. | |
| SoxS (Regulatory protein) | Transcriptional activator of the superoxide response regulon [41][60]. It also facilitates binding of RNA polymerase to the micF and the nfo promoters [42][61]. | |
| SulA (Cell division inhibitor) | Component of the SOS system and an inhibitor of cell division [43][62]. | |
| UmuD | Involved in UV protection and mutation. Poorly processive, error-prone DNA polymerase involved in translesion repair [44][63]. | |
| TrfA (Plasmid replication initiator protein) | Required for the initiation of plasmid DNA replication, along with host-derived DnaA and other host proteins [45][64]. | |
| RepE (Replication initiation protein) | Replication initiator in the monomeric form, and autogenous repressor in the dimeric form [46][65]. | |
| CcdA (antitoxin) | Antitoxin component of a type II toxin-antitoxin (TA) system which inhibits the post-segregational killing (PSK) of plasmid-free cells [47][66]. | |
| Bacillus subtilis Lon | SwrA | Modulator of the two-component system DegSU; it is important for swarm motility [48][67]. |
| Yersinia pestis Lon | YmoA (Yersinia modulator A) | Small histone-like protein which is required for Yersinia T3SS induction [49][68]. |
| RsuA (Ribosomal small subunit pseudouridine synthase A) | Responsible for synthesis of pseudouridine from uracil-516 in 16S ribosomal RNA [50][69]. | |
| Fur (Ferric uptake regulation protein) | Acts as a repressor, employing Fe2+ as a cofactor to bind the operator of the iron transport operon [51][70]. | |
| Caulobacter crescentus Lon | Cell-Cycle-Regulated DNA Methyltransferase (CcrM) | Regulates the methylation of chromosomal DNA and cellular differentiation [35][54]. |
4. Role of Redox Switch in Modulating Lon-MEDIATED Proteolysis
The Enterobacteriaceae family members, e.g., Escherichia coli, Shigella, Salmonella, Serratia and Yersinia pestis are mostly symbionts dwelling in the gut of the host [52][71]. These bacteria are some of the commonly encountered pathogens in clinical microbiology and cause a wide range of diseases. They are facultative anaerobes that live in both harsh aerobic and anaerobic environments. The Lon protease redox switch seems to play a vital role in enabling the facultative lifestyle of these organisms. Nishii et al. [4] reported that the conserved cysteine residues in Lon proteases of these bacteria act as a redox switch, which changes the size of the exit pore of the P-domain ring to regulate the proteolytic activity depending on the availability of oxygen. In Escherichia coli Lon, only Cys617 and Cys691 are sensitive to redox conditions, whereas the other cysteine residues seem to be impervious to it. In an aerobic environment, the microbes constantly encounter oxygen and oxidants, as well as reactive oxygen species that are produced as byproducts of aerobic respiration. This oxidative stress leads to the production and accumulation of damaged and misfolded proteins. The high activity level of the oxidized form of Lon protease helps to eliminate the intracellular faulty proteins. Although the high activity level of Lon may pose a threat to the native cellular proteins, their replenishment is swift in the oxygen-rich environment, as aerobic respiration rapidly produces the ATP required for protein synthesis. On the other hand, upon entering the host’s body, these bacteria live in the intestine, and primarily, in the colon, the regions that are almost free of oxygen. These anaerobic environments slow the rate of cellular protein synthesis due to inefficient production of ATP, thereby reducing the amount of improperly synthesized and/or folded proteins. Thus, the low activity level of the reduced form of Lon protease is suitable for anaerobic environments. Consequently, these pathogenic bacteria can fine-tune the activity of Lon protease by an extraordinary mechanism to live robustly both inside and outside the host body [4].
