Ovarian cancer (OC) is gynecological cancer, and diagnosis and treatment are continuously advancing. Next-generation sequencing (NGS)-based diagnoses have emerged as novel methods for identifying molecules and pathways in cancer research. The NGS-based applications have expanded in OC research for early detection and identification of aberrant genes and dysregulation pathways, demonstrating comprehensive views of the entire transcriptome, such as fusion genes, genetic mutations, and gene expression profiling. Coinciding with advances in NGS-based diagnosis, treatment strategies for OC, such as molecular targeted therapy and immunotherapy, have also advanced. Immunotherapy is effective against many other cancers, and its efficacy against OC has also been demonstrated at the clinical phase.
- next-generation sequencing
- ovarian cancer
- immunotherapy
1. Introduction
23. Immunotherapy in OC
In cancer research, immunotherapy enhances the immune response such that the patient’s immune cells can kill cancer cells. It is effective against several tumors and has innovative therapeutic potential to replace chemotherapy as the next-generation treatment for gynecological cancers [26][81]. Immunotherapy can be categorized into active and passive types. Active immunotherapy directly targets specific cancer antigens, and this involves treatment with immunostimulatory vaccines, genetically modified to recognize target antigens or chimeric antigen receptor T cells (CAR-T). Passive immunotherapy involves treatment with cytokines or checkpoint inhibitors, which indirectly activate a patient’s immune response [27][82]. These treatments are innovative, but according to the results of clinical trials, only some cancers can be treated, and side effects, such as cytokine syndrome and decreased immune function, can occur [26][28][81,83].2.1. Immune-Stimulating Peptide Vaccines
3.1. Immune-Stimulating Peptide Vaccines
Vaccine-mediated immunostimulatory therapy involves the use of a specific antigenic vaccine of the tumor, which is an immunotherapeutic agent that suppresses tumor growth or cancer recurrence by inducing an immune response [29][30][84,85]. Peptide vaccines consist of DNA vectors expressing dendritic cells, recognized by autologous tumor-specific antigens or tumor-specific peptides that induce immune activation, and the activity of which can be enhanced by various immune modulators [31][32][86,87].23.1.1. P53 Peptide Vaccine
The most common mutant gene in human cancers is p53, with nearly 50% of OCs having p53 mutations [33][88]. Because p53 mutations mainly increase the expression and stability of the p53 protein, the amount of p53 in cancer cells is higher than that in normal cells [34][89]. Therefore, a p53 peptide vaccine could be a targeted therapeutic strategy for various cancer cells. It has been reported that p53-synthetic long peptide (p53-SLP) vaccine treatment in OC patients significantly induced p53-SLP-specific T-cell responses [35][90]. In addition, low-dose cyclophosphamide pre-treatment in patients with recurrent OC improved the immunogenicity of the p53-SLP vaccine, by enhancing the activity of regulatory T cells [36][91]. In a clinical study on platinum-resistant OC, p53 vaccine and gemcitabine significantly increased the reactivity of T cells, leading to better survival [37][92]. Recently, studies on the combination of various immunomodulators and p53 vaccines have been conducted to improve biochemical sensitivity and immune responses [38][39][93,94].23.1.2. Wilms’ Tumor 1 (WT1) Peptide Vaccine
WT1 is often detected in various cancers, including leukemia, lung cancer, breast cancer, thyroid cancer, melanoma cancer, and OC [40][95]. Mouse and human WT1 genes have high homology, and hence, a murine model was used for WT1 vaccine development [40][95]. After confirming that the WT1 vaccine inhibited the growth of cancer cells by inducing a strong immune response, a WT1 vaccine using serum from cancer patients was evaluated for the first time [40][95]. HLA-A*24:02-restricted WT1-targeting vaccine promoted peptide-specific humoral immunity in refractory OC patients; therefore, WT1 levels can be used as a potential prognostic marker [41][96].23.1.3. New York Esophageal Squamous Cell Carcinoma-1 (NYESO-1) Peptide Vaccine
NYESO-1 is a marker expressed in various cancer cells, including OC, and it is a significant target for vaccines [42][97]. Vaccine-mediated CD4+ and CD8+ T-cell responses, and the application of NY-ESO-1+ lymphocytes, have been reported [43][98]. The modified NY-ESO-1 vaccine rapidly induced a consistent and safe immune response in most vaccinated OC patients [44][99]. A recent study reported that the NYESO1 vaccine linked to the secretin-penetratin peptide induced a stronger and more specific T-cell immune response in a mouse animal model [45][100].23.1.4. Glypican-3 (GPC3)-Derived Peptide Vaccine
GPC3, a heparan sulfate proteoglycan bound to the cell membrane by glycosylphosphatidylinositol, is specifically expressed in liver cancer, OC, lung cancer, and melanoma [46][47][101,102]. A recent report showed that the GPC3 vaccine improved the survival rate of chemoresistant OCs after chemotherapy [48][103]. In addition, it has been reported that adjuvant miRNA enhances the effect of GPC3 vaccine, and that the combination of miR-375, 193a, and 1128 can be used as predictive biomarkers [49][104]. The GPC3 vaccine was used in combination with CAR-T therapy, to significantly enhance the therapeutic effect against OC, suggesting that the GPC3 vaccine could be used in combination with several cancer treatments in the future [50][105].23.1.5. Dendritic Cell (DC)-Based Peptide Vaccine
DCs are antigen-presenting cells of the immune system and are widely recognized as important cell types that can initiate antitumor responses [51][106]. DC-based vaccines are a novel strategy for effectively treating cancer patients, and hence, various clinical studies have been conducted using DC-related responses in cancer patients. Correll et al. reported that a DC vaccine could inhibit the activity of regulatory T cells in the blood of melanoma patients, and subsequently restore T-cell activity [52][107]. As a result of these observations, DC vaccines were evaluated in several carcinomas. Autologous DC-based vaccines with tumor lysates after chemotherapy in OC patients have also been reported to successfully reduce the rate of cancer progression and improve survival [53][108]. In addition, DC vaccines related to neoantigen peptides are being developed as clinical candidates for immune enhancement [54][109]. DC-based vaccines have great potential to improve the treatment of OC patients, and the advantage of using the immune system to induce a more sustained response, compared with that during cytotoxic chemotherapy [55][110]. However, further clinical studies of a cohort of patients with OC using DC vaccines are needed to confirm its safety and efficacy.2.2. Blockade of Checkpoint
3.2. Blockade of Checkpoint
Checkpoint inhibitors are widely used to treat refractory tumors, including OC. Immune checkpoints are generally regulated by negative feedback inhibition, and they protect the host from autoimmunity and maintain self-tolerance by regulating the responses of various immune cells [56][57][111,112]. When activated T-cells recognize and bind to specific markers of tumor cells, immune checkpoints rapidly regulate the immune response through the T-cell receptor (TCR) signaling pathway [58][113].23.2.1. Cytotoxic T-lymphocyte Antigen 4 (CTLA-4) Inhibitor
CTLA-4 inhibitors are widely used as immune checkpoint blockers to induce an immune response. PHI-101, a checkpoint kinase 2 inhibitor, showed a tumor-reducing effect on platinum-resistant, recurrent OC [59][114]. PHI-101 therapy was assessed in a phase IA clinical trial, and a phase II clinical trial has been recommended for the same. Ipilimumab, under the trade name Yervoy, is a CTLA-4 antibody generally used in the treatment of various cancers [60][115]. However, the anti-CTLA-4 antibodies need improvement, because immunotoxicity has often been reported in the liver, gastrointestinal tract, and endocrine system within the first few weeks of treatment [61][116].23.2.2. Programed Cell Death Protein 1 (PD-1) and Programed Cell Death-Ligand 1 (PD-L1) Inhibitors
PD-1, a co-inhibitory receptor, inhibits T-cell activation, suggesting that it is a target for immunotherapy in cancer cells. PD-1 has two types of potential ligands: PD-L1 and PD-L2. PD-L1 is mainly expressed in most hematopoietic cells and vascular endothelial cells, whereas PD-L2 is expressed in some macrophages and dendritic cells [62][117]. PD-L1 is generally found in various malignancies, including OC [63][64][118,119]. PD-1–PD-L1 interaction normally inhibits the survival, proliferation, and functions of T cells [65][120]. Blocking the interaction of PD-1 with PD-L1 is widely used clinically for the treatment of tumors, and this strategy is less toxic than the use of CTLA-4 inhibitors, such as ipilimumab [66][121]. Pembrolizumab, also known as Keytruda, is a PD-1 inhibitor, and it has been effective in patients with recurrent OC [67][68][122,123]. A recent study showed that the combination of pembrolizumab and PEGylated liposomal doxorubicin has a potential therapeutic effect on platinum-resistant OC [69][124]. The combination of pembrolizumab with bevacizumab dramatically decreased the serum CA-125 level and regression of recurrent masses, with no marked side effects [70][125]. Nivolumab, under the trade name Opdivo, is a human monoclonal antibody approved for the treatment of several malignancies; it enhanced the anticancer activity of T cells by blocking the PD-1 and PD-L1/L2 pathways [63][71][72][118,126,127]. Monotherapy with nivolumab exhibited low tumor specificity and response in platinum-resistant OC [71][126]. Co-administration of nivolumab with various adjuvants has shown optimistic trends in the treatment of various cancers, including OC. In addition, the combination of nivolumab with the anti-CTLA4 antibody, ipilimumab, has been reported to improve the overall response to OC [73][128]. Atezolizumab (tecentriq; MPDL3280A) is an immunoglobulin mAb, that selectively interacts with PD-L1 in cancer cells, in the tumor microenvironment, and reactivates suppressed T cells to kill malignant tumors [74][129]. As PD-L1 expression is highly detectable in OC specimens, atezolizumab has been attracting attention as a potential immunotherapeutic agent. In addition, a combination of atezolizumab and other immunotherapeutic agents showed preliminary clinical activity in patients with OC [75][130]. For example, the combination of atezolizumab and bevacizumab induces a sustained response in some patients with platinum-resistant OC [76][131]. Abelumab, a monoclonal antibody capable of mediating antibody-dependent cytotoxicity, is a checkpoint inhibitor that interacts with PD-L1, especially when administered in combination with chemotherapeutic agents [77][78][132,133]. Several clinical studies have shown that avelumab monotherapy is effective in Merkel cell carcinoma (MCC) and urothelial cancer [79][134]. In addition, it has been reported that combination therapy with avelumab and axitinib increased the survival rate of urothelial cancer patients and showed significant activity when combined with docetaxel [79][134]. Several combination strategies have recently been evaluated, and further studies are underway to improve the response rate of avelumab in patients with OC [80][81][82][135,136,137].2.3. Chimeric Antigen Receptor T (CAR-T) Cells
3.3. Chimeric Antigen Receptor T (CAR-T) Cells
CAR-T cells are genetically modified patient-derived immune cells designed to activate the immune response by recognizing specific surface antigens on cancer cells. The inhibition of histone deacetylase (HDAC) activity plays an important role in CAR-T cell immune recognition. Co-treatment with sodium valproate (VPA), a representative HDAC inhibitor, and CAR-T enhanced the immune recognition of CAR-T cells in OC [83][138]. The identification of specific antigens overexpressed in cancer cells is an important strategy in CAR-T therapy. The most common target antigens for CAR-T in OC are mesothelin, mucin 16 (MUC16), folate receptor α, and human epidermal growth factor receptor 2 (HER2) [84][139]. Mesothelin is a new antigen that can be a target of CAR-T and is overexpressed in various cancers, including OC. However, it is also expressed in some normal tissues and has the disadvantage of causing off-target effects [85][140]. Mouse studies have shown that treatment with mesothelin-induced CAR-T cells is a potential therapy for OC. It was able to significantly prolong the survival of mice with OC [86][141]. Mucine 16 (MUC16), a glycoprotein of the mucin family, is expressed in various tumor cells and is involved in the proliferation and metastasis of cancer cells [87][142]. MUC16 is strongly expressed in most OCs and known well as a tumor marker (CA125), because it is cleaved and released from peripheral blood, suggesting that MUC16 is an ideal antigenic target for CAR-T. It has been reported that PD1-anti-MUC16 CAR-T cells have more potent anticancer activity than single MUC16-CAR-T cells in OC animal models [88][89][143,144]. The clinical application of MUC16 is still lacking due to several limitations, but immunotherapeutic studies using CAR-T cell construction are ongoing [90][145]. Folate receptor-α (FRα) protein is expressed at low levels in normal cells, specifically in OC [91][146]. T-cell activation using CAR targeting with FRα has been evaluated for use in OC treatment [92][147]. Recently, CAR-modified, cytokine-induced killer cells with FRα, enhanced anticancer immunity against OC [93][148]. Human epidermal growth factor receptor 2 (HER-2) is overexpressed in breast cancer and OC [94][149]. One study reported that radiolabeled pertuzumab for HER2 imaging enables rapid and unambiguous delineation of OCs overexpressing HER2 [95][150]. The suppression of HER2 using shHER2-RNA treatment with cisplatin also enhanced the anticancer effect of OC [96][151]. The study of HER2-interact synthetic Notch CAR cells has been investigated in a mouse model, and it is expected that clinical therapeutics for HER2-CAR-T cells will also be developed in the near future [97][152] (Table 12 and Figure 12).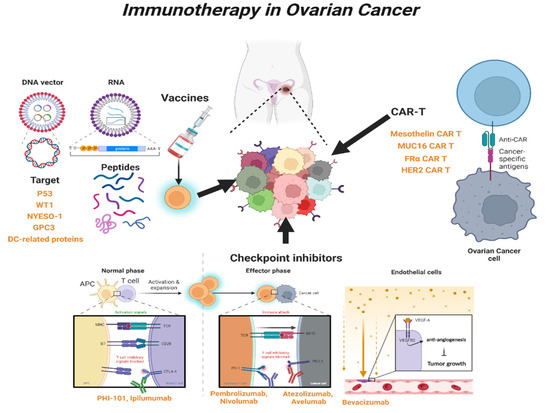
| Type of Mechanism | Agents | FDA Approval |
Combination with | Ref. |
|---|---|---|---|---|
| Peptide vaccines | ||||
| P53 | P53-SLP vaccine | no | Cyclophosphamide; Gemcitabine | [35][36][37][90,91,92] |
| WT1 | WT1 vaccine | no | [40][41][95,96] | |
| NYESO-1 | NYESO-1 vaccine | no | Secretin-penetratin | [42][43][44][45][97,98,99,100] |
| GPC3 | GPC3 vaccine | no | miR-375, miR-93a, miR-1128; CAR-T cell | [48][49][50][103,104,105] |
| DC | DC protein vaccine | yes | [52][107] | |
| DC | Neoantigen vaccine | no | [53][108] | |
| Checkpoint inhibitor | ||||
| CTLA-4 | PHI-101 | yes | [59][114] | |
| CTLA-4 | Ipilimumab | yes | [60][115] | |
| PD-1 | Pembrolizumab | yes | Doxorubicin; Bevacizumab | [67][68][69][70][122,123,124,125] |
| PD-1 | Nivolumab | yes | Ipilimumab | [71][73][126,128] |
| PD-L1 | Atezolizumab | yes | Bevacizumab | [75][76][130,131] |
| PD-L1 | Avelumab | yes | Axitinib; Docetaxel; Doxolubicin | [79][80][81][134,135,136] |
| PD-L1 | Avelumab | yes | Carboplatin + Paclitaxel | [82][137] |
| CAR-T | ||||
| Mesothelin | MSTL-CAR-T | no | [86][141] | |
| MUC16 | PD1-MUC16-CAR-T | no | [89][144] | |
| FRα | FRα-CAR-T | no | [92][147] | |
| FRα | FRα-CAR-T | no | Cytokine-inducing killer cell | [93][148] |
| HER2 | HER2-CAR-T | no | Pertuzumab | [95][150] |
| HER2 | HER2-CAR-T | no | Synthetic Notch | [97][152] |
