Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 5 by Jessie Wu and Version 4 by Jessie Wu.
Extracellular vesicles (EVs) are cell-derived membrane vesicles that represent an endogenous mechanism for intercellular communication. The original classification distinguished exosomes (nano-sized vesicles with a diameter in the range of 30 to 120 nm), originating from the formation of multivesicular bodies, microvesicles, which are formed by cell membrane budding, and apoptotic bodies, derived from dying cells.
- red blood cell extracellular vesicles
- extracellular vesicles
- drug delivery
1. Introduction
Human red blood cells (RBCs) are terminally differentiated, enucleated, and very versatile cells that have been used for drug delivery systems since the end of the last century. Most of their applications as drug delivery systems have been extensively reviewed in [1][2][3]. RBCs have a flexible biconcave shape with a diameter of 7.5–8.7 μm and thickness of 1.7–2.2 μm, their membrane mainly contains 60% phospholipids, 30% cholesterol, and 10% glycolipids [4]. Moreover, the RBC membrane also contains various proteins, such as peripheral and integral proteins, which can be classified into three groups: cytoskeletal proteins (e.g., spectrin, actin, protein 4.1), integral structural proteins (e.g., band 3, glycophorins), and anchoring proteins (e.g., ankyrin, protein 4.2) [5]. All these features confer on RBCs a high degree of plasticity and elasticity that in vivo allows them to pass across very small capillaries and exert their transporting function, while in vitro they can be exploited for therapeutic purposes. Hemoglobin is the major cytosolic protein in intact RBCs; the cytoplasmic fraction also contains several antioxidant and metabolic enzymes needed for the RBC’s own metabolism. These proteins can release adenosine triphosphate (ATP) and NO into the intracellular environment [6]. RBCs are the major vesicle-secreting cells in blood circulation. During their 120-day lifespan, RBCs lose ∼20% of their hemoglobin content and membrane integrity during physiological vesiculation [7]. The physiological aging of RBCs accelerates vesicle generation. Indeed, vesiculation is one of the most important mechanisms by which RBCs eliminate harmful substances accumulated throughout their lifespan [8][9]. Under normal physiological conditions, RBC-derived EVs constitute 7.3% of EVs in whole blood, indicating that RBCs are one of the main sources of EVs in peripheral blood [10][11].
RBC membrane vesiculation is a homeostatic process activated in response to impaired signaling machinery; it can be induced by ATP depletion, calcium loading, lysophosphatidic acid exposure, oxidative stress, endotoxins, cytokines, complement, and high shear stress [12]. Red blood cell-derived extracellular vesicles (RBCEVs) can be divided into subpopulations, such as exosomes and microvesicles.
Exosomes can be produced during the reticulocyte or erythroid precursor stage and maintained until the mature RBC stage [13]. Erythropoiesis is a long process that starts with a myeloid precursor and ends with reticulocytes maturing into erythrocytes. This terminal differentiation is accompanied by a cellular remodeling that leads to the disappearance of intracellular organelles, the elimination of membrane and cytoplasmic content, and the acquisition of the typical cellular biconcave form. Blank et al. proposed for the first time that exosome biogenesis and secretion contribute to the net loss of the cell surface membrane via selective vesicular membrane secretion [14]. Exosomes derived from reticulocytes are generated via the typical endosomal pathway of nucleated cells after the plasma membrane has invaginated to form the early endosome. The early endosome subsequently matures into a late endosome that evolves into multivesicular bodies carrying intraluminal vesicles [13].
On the contrary, vesicles may form during the normal aging of circulating erythrocytes due to complement-mediated calcium influx, plasma membrane budding, and subsequent vesicle shedding [15]. The biogenesis and release of microvesicles (MVs), which originate by membrane budding, is an integral part of red blood cell physiology and is linked to their maturation and aging. Indeed, by releasing MVs, the erythrocyte can eliminate damaged components that could also trigger hemostatic and immunological reactions [13]. Primarily, the formation of MVs from red blood cells is triggered by damaged hemoglobin, protein oxidation, degradation caused by senescence, and cytoskeletal binding of ankyrin to band 3. Another mechanism involved in the production of microvesicles is the alteration of phospholipid distribution in the lipid bilayer. Indeed, certain enzymes such as scramblase, calpain, and some proteases can be activated by oxidative stress or an increased influx of calcium ions, thus leading to the inhibition of flippase with the consequent exposure of phosphatidylserine, usually expressed in the inner bilayer layer, or leading to the proteolytic degradation of the cytoskeleton and the consequent aggregation of band 3 [13].
2. Red Blood Cell-Derived Extracellular Vesicles Composition
RBCEVs are generally visualized as round vesicles of 100–200 nm and comprise phospholipids, proteins, cholesterol, lipid rafts, hemoglobin, and acetylcholinesterase [9]. Although RBCEVs are derived from RBCs, their membrane compositions and internal contents are not exactly the same. Indeed, the final composition of RBCEVs is supposed to be dependent on the resealing and stimulating conditions. Indeed, EVs produced from RBCs are reported to be different when produced naturally in vivo, released ex vivo during blood bag storage, or produced in vitro by chemical treatments [16]. According to Yang et al., RBCEVs lack cytoskeletal components such as spectrin and actin, whereas they are relatively enriched in connexins and lipid raft markers [17]. Thangaraju et al. compared about 30 papers reporting the composition of RBCEVs obtained after different procedures of isolation/production and in different physiological/pathological conditions [13]; while Chiangjong et al. made a deep comparison of the components of the parental cells (RBCs) and those of the derived vesicles (RBCEVs) [9]. Taken together, these data suggest that the different conditions or production methods can affect the final composition; however, some similarities can be found and are reported in the next paragraph.
Extracellular vesicles are highly enriched with proteins with different functions, such as tetraspanins (CD9, CD63, CD81, and CD82), which are involved in cell penetration, invasion, and fusion events; heat shock proteins (HSP70, HSP90), which are involved in antigen anchoring and presentation; and MVB formation proteins (Alix, TSG101), which are involved in exosomal release [18]. Additionally, cytoskeletal proteins (e.g., actin), denatured hemoglobin, proteins 4.1, 4.2, enzymes such as carbonic anhydrase, anion transport proteins (Band 3), glycoproteins (e.g., CD235a), membrane-associated proteins such as stomatin and flotillin, and CD47, which inhibits phagocytosis by interacting with macrophage signal regulatory protein alpha [19]. Whereas, in the membrane lipid part, researechers find phosphatidylserine (PS), which is often found in the outer membrane layer and has a role in biogenesis (see next paragraphs), phosphatidylethanolamine, phosphatidic acid, diacylglycerol, and cholesterol [9][13]. The most striking and interesting discovery is that these vesicles have different types of miRNAs inside them, which can be harnessed and directed towards a specific target to exert their action as modulators of gene expression [13][20]. Among nucleic acids, DNA is obviously lacking, while miRNAs are present in huge amounts [21]. About 78 different miRNAs were found, with miR-125b-5p, miR-4454, and miR-451a being the most abundant [20][21]. On this basis, Sun et al. proposed that under certain conditions, RBCEVs can send miRNA to recipient cells to exert their function [22].
3. Red Blood Cell-Derived Extracellular Vesicles Production under Physiological and Pathological Conditions
RBCEVs are naturally produced during the RBC’s life and are released into circulation, where they can interact with numerous tissues and/or cells to influence their functions. RBCEVs can play roles both in physiological and pathological conditions, but if the RBCEV level in healthy states is assumed to be normal, their level is supposed to be raised with aging or under other diseased or stressed conditions [17].
The first physiological function that has been discovered is during the maturation of RBCs. Indeed, they remove excess proteins and membranes such as transferrin receptors, acetylcholinesterase, and hemoglobin via vesiculation processes during the passage from reticulocyte to mature RBC [23][24]. RBCEVs can protect RBCs by clearing dangerous molecules and preventing their early clearance from circulation [25][26]. In addition, RBCEVs partly inherit the role of RBCs. RBCEVs are critical for communicating with endothelial cells to regulate NO and O2 homeostasis. Indeed, under storage conditions, RBCEVs react faster with NO than intact red blood cells, causing strong vasoconstriction [6][27]. RBCEVs can affect a variety of immune cells [28][29][30]. RBCEVs can promote the production of pro-inflammatory cytokines (interleukin (IL)-2, -7, and -15) and tumor necrosis factor-alpha (TNFα) by interacting with macrophages [30]. RBCEVs also increased proliferation in CD4+ and CD8+ T cells by influencing antigen-presenting cells [28]. Finally, RBCEVs may also have a role in coagulopathy [13]. Some authors have suggested that PS exposed to the external membrane layer can contribute to coagulation by triggering the intrinsic pathway. However, other authors reported an anti-coagulant role of RBCEVs mediated by their interaction with protein S, protein C, and fibrinogen [31][32].
As mentioned before, RBCEVs have been known as key regulators of various physiological and pathological processes, including coagulation, inflammation, and also atherosclerosis and thrombosis [33]. Moreover, there is strong evidence that the plasma concentrations of RBCEVs are elevated in the development of cardiovascular-related disease, which can further lead to vascular dysfunction [34]. Furthermore, RBCs infected by Plasmodium falciparum are able to transfer DNA (drug resistance and fluorescent protein genes, etc.) through the RBCEVs, helping the parasite survive times of stress [35]. RBCEVs in disease states often have pro-inflammatory and pro-coagulant effects. Different studies have shown that elevated levels of the circulating pro-coagulant RBCEV lead to increased thrombotic and hypercoagulable states in sudden nocturnal hemoglobinuria (PNH) and hemolytic disease [36][37]. RBCEVs can finally interfere with NO homeostasis by increasing ROS production, which can also lead to endothelial dysfunction [38].
4. Red Blood Cell-Derived Extracellular Vesicles Production and Isolation for Therapeutic Purposes
RBCs are an excellent source for the production of EVs for drug delivery. RBCEVs have several distinct advantages compared with other types of cell-derived and artificially produced EVs [9]. Synthetic nanovesicles created from biomimetic phospholipid bilayers resulted in several improvements, such as increased solubility, prolonged action, reduced toxicity, and lesser adverse effects, by mimicking EV properties [39][40]. However, the issues limiting the utility of those synthetic nanocarriers are immunogenicity and higher clearance by phagocytic cells [41]. Conversely, RBCEVs have been proven extremely safe thanks to their higher biocompatibility compared to synthetic ones. In addition, compared to EVs derived from nucleated cells, RBCEVs have a lower risk of horizontal gene transfer because RBCs lack both nuclear and mitochondrial DNA; thus, RBCEVs are particularly advantageous for delivering genetic material, e.g., RNA molecules and long mRNA molecules [42]. Furthermore, RBCEVs can escape macrophage clearance through the binding of CD47 to inhibitory receptor signal regulatory protein α SIRPα), thus preventing RBCEV clearance via endogenous elimination [19]. Finally, RBCEVs produced by O blood type cells may be used in allogeneic individuals, and the blood cells are easy to obtain from donors. Thus, RBCEVs represent an excellent delivery system for carrying drugs to cellular targets with cost-effectiveness, non-immunogenicity, and high stability and biocompatibility [9].
Nevertheless, the main limitations remain the way of scaling up RBCEVs production to increase the yield and the way to load cargo into EVs. These will be discussed in detail in the following paragraphs.
Methods for Scaling up Red Blood Cell-Derived Extracellular Vesicles Yield
Several stimuli have been shown to stimulate RBCEVs; Chiangjong and colleagues summarized them very well in a table reporting a list of chemical reagents, oxidative molecules, and storage conditions for RBCEV production [9]. In the next sub-paragraphs, researchers will discuss the most used methods for producing high amounts of RBCEVs in vitro or ex vivo; these have been outlined in Figure 1.
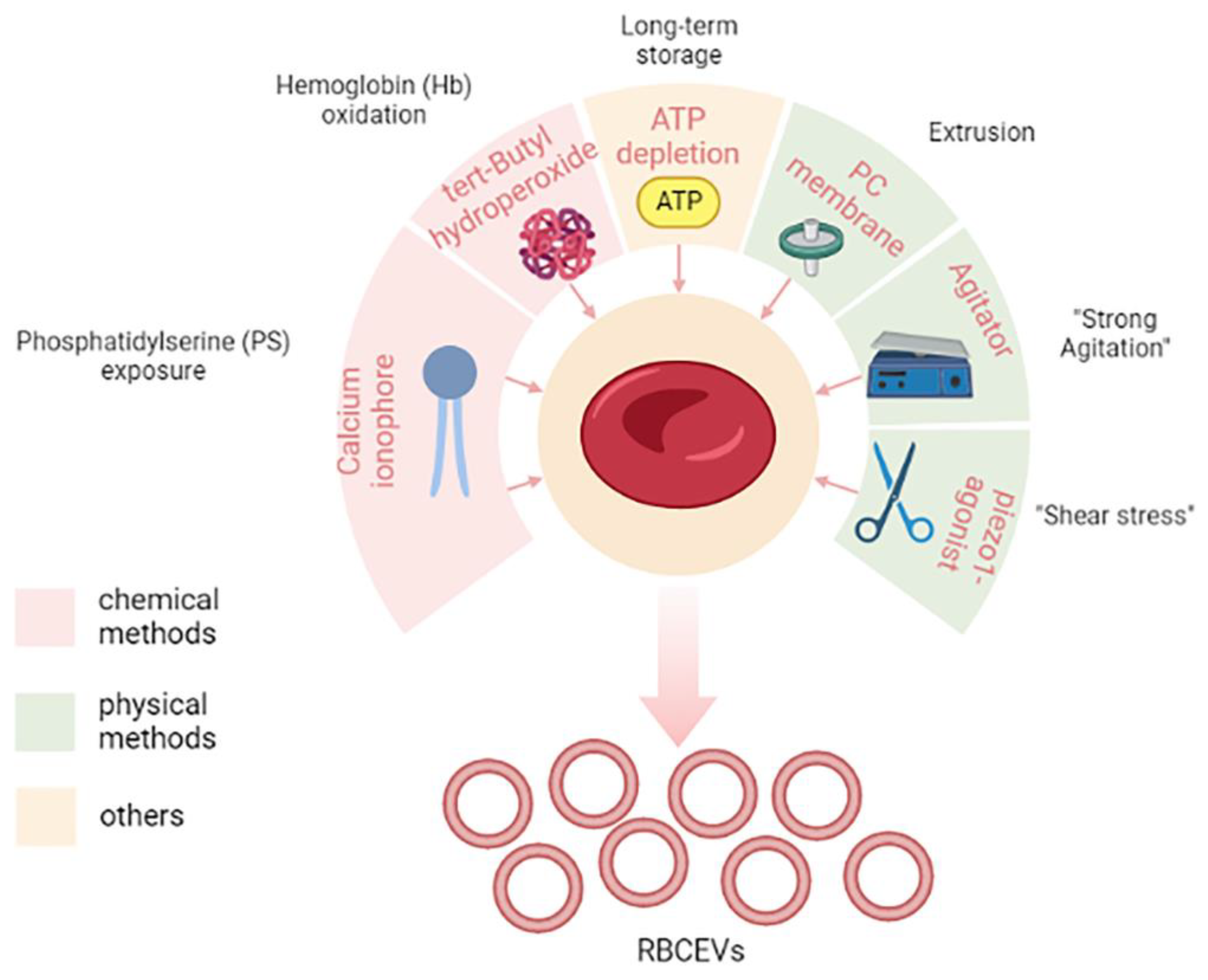
Figure 1. Methods for increasing the yield of RBCEVs production. In the figure, different chemical and physical methods are shown. In particular, molecules and/or mechanical stimuli are depicted together with their effect on the cells that give rise to RBCEVs release. Long-term storage is a particular condition that cannot be included in either the chemical or physical methods. Created in Biorender.com, accessed on 5 December 2022.
Among chemical methods, the most used is the in vitro stimulation with calcium ionophore. Usman and colleagues provided a lab-based approach to treat isolated RBCs with calcium ionophores that can stimulate the release of RBCEVs [42]. The protocol also envisaged the purification by means of several rounds of low-speed centrifugations, filtration, and ultracentrifugation steps. This appeared to be a feasible, cost-effective, and high-yielding approach, thus making it one of the most used in laboratory settings. Large-scale amounts (1013–1014) of RBCEVs can be isolated from RBCs (per unit, 200 mL of blood) when treated with calcium ionophore, which is thus a promising and scalable strategy to obtain EVs in vitro.
Calcium ionophore is supposed to induce vesiculation by activation of PS exposure to the outer surface membrane, thus leading to membrane budding and microvesiculation [9]. Other chemicals can mimic the same process, such as phorbol 12-myristate 13-acetate and lysophosphatidic acid [9].
Another method and/or process that induces vesiculation is the induction of oxidative stress, for example, by means of tert-butyl hydroperoxide, which leads to increased osmotic fragility and Hb oxidation [43].
Finally, it has also been reported that long-term storage, like in blood banking conditions, can stimulate the production of RBCEVs [9][44]. This has obvious consequences in transfusion medicine.
Nowadays, it is not completely understood whether and how different kinds of stimuli can affect RBCEVs’ properties and composition, so characterization is an important step at the end of the production.
In addition to chemical methods, there are different so-called “physical” vesiculation methods that encompass several different methods that can mimic “shear stress”. One of the most used physical vesiculation methods is extrusion, but recently other methods have been proposed.
Gangadaran and co-workers optimized a scaling-up strategy to produce RBCs exosome mimetics (RBC-EMs) based on extrusion [45]. RBC-EMs produced by this method have similar characteristics as RBC exosomes. To obtain RBC-EMs, RBCs were purified from fresh blood and diluted in phosphate-buffered saline. The diluted RBCs were passed through a 1 µm polycarbonate membrane four times by using an extrusion set. After this physical stress, the obtained samples were purified by ultracentrifugation as before. This seems another promising and scalable strategy to obtain RBCEVs and allowed 130-fold higher production yield in terms of particle numbers compared to native exosome release.
Recently, Erytech Pharma patented a novel vesiculation method to produce large-scale RBCEVs also based on “shear stress”. In particular, after the loading procedure (see the next paragraph for a focus on loading procedures), they put loaded RBCs under “strong agitation” for several hours, and this induced the production of RBCEVs. This method will be discussed more deeply in the section dedicated to patents (see “State of the art of the technology from the industrial side”).
More recently, other authors proposed another method to induce mechanical stimuli for vesiculation, which is Piezo1 stimulation [46]. Briefly, Sangha and collaborators found that treating 6% hematocrit RBCs with 10 µM piezo1 agonist yoda1 for 30 min maximized RBCEV yield until 1012 particles/mL. This paper was available as a pre-print at the time of its writing.
5. Methods for Cargo Loading in Red Blood Cell-Derived Extracellular Vesicles
Growing evidence shows that RBCEVs can not only deliver biological information but also different kinds of drugs, nucleic acids, and proteins. Nevertheless, another limitation is exactly the way cargo is loaded. Han et al., in 2021, revised the whole methods for loading cargo into different kinds of EVs. He divided the methods between “cell-based” and “non-cell-based” [47]. Cell-based methods, also called pre-loading, are based on the indirect encapsulation of therapeutic cargo into the donor cell before the production of EVs. In this approach, different cargoes can be encapsulated into the donor cells essentially by simple incubation and/or transfection. During vesiculation, cargoes are then packaged into EVs and ultimately delivered to recipient cells for therapeutic use [48]. This method provides a convenient and effective way for loading biological materials and drug therapies into EVs [47]. On the contrary, the non-cell-based loading approach involves the direct loading of chemical or biomolecules into already-produced EVs and can be performed through electroporation, sonication, incubation, and/or transfection [49]. Thus, non-cell-based EV loading methods incorporate therapeutic cargo into EVs after isolation and/or production, and for this reason, it is also known as “post-loading”. Different siRNAs, miRNAs, proteins, CRISPR/Cas9, hydrophobic compounds, and anticancer drugs can be loaded into EVs through non-cell-based loading [47]. These loading methods can be further classified into passive loading and active loading [47]. Passive loading involves loading the therapeutic cargo into EVs through diffusion, whereas active loading consists of the disruption of EV membranes through electroporation or sonication, allowing entry into the EVs.
In this revisewarch, which is focused on RBCEVs, researchers prefer to talk about pre-loading and post-loading methods. As mentioned, RBCs have been used longer for drug delivery [2][3][50]; indeed, they possess numerous features that make them ideal candidates for this purpose: (i) are biodegradable; (ii). are available in large quantities; (iii) can circulate for long periods of time (months); (iv) have a large capacity; (v) are not toxic or immunogenic; (vi) have a long in vivo life span; and (vii) several procedures exist to encapsulate a wide range of molecules inside them. Regarding this last point, many loading methods (electroporation, drug-induced endocytosis, osmotic pulsing, and hypotonic hemolysis) have been set up over the years and are mostly based on the transient opening of pores across the cell membrane, as detailed in Rossi et al. and Magnani et al. [50][51].
RBCs possess, in fact, the remarkable capacity for reversible shape change and for reversible deformation, allowing the opening of pores (20–50 nm in diameter) large enough to be crossed by externally placed macromolecules. Among the above-mentioned methods, the hypotonic hemolysis one is what allows for obtaining engineered RBCs with the most suitable characteristics for biomedical applications. In turn, it includes different procedures, such as dilutional, preswell dilutional, and dialysis ones, which can be opportunely selected by the researchers according to them. However, all these procedures are based on the same physical-chemical features of RBCs. When placed in the presence of a hypotonic solution, RBCs increase in volume, and their morphology is converted to spherocytes; since RBCs have little capacity to resist volume increases when placed in solutions of appropriate mOsm/kg, the membranes rupture with the formation of large pores, permitting the influx of molecules of interest. By raising the salt concentration to its original level, the membranes can be resealed and the added substances entrapped in erythrocytes [52][53]. It must be emphasized that when a procedure moves from the laboratory to the clinic, the availability of appropriate equipment based on an appropriate method becomes very important. To date, two companies, ERYtech Pharma S.A. and EryDel S.p.A., have ongoing phase III clinical trials (ClinicalTrials.gov identifiers: NCT03674242 and NCT03563053, respectively) based on engineered RBCs obtained by dialysis or the preswell dilutional method, respectively. Their equipment allows them to carry out fully automated loading procedures in perfect sterility and apirogenicity conditions, as is needed in clinical use.
Regarding the post-loading methods, the most used for RBCEVs are electroporation and transfection. Both of them have been widely used for the encapsulation of nucleic acids. Iconic examples are represented by Usman et al. that used electroporation [42] and Peng et al. that preferred the transfection method [54]. Several other examples are reported in the next paragraph, which focused on the therapeutic applications of RBCEVs.
The different cargo methods are outlined in Figure 2.

Figure 2. Methods for cargo loading in RBCEVs. Loading methods have been divided into two main classes, namely pre-loading and post-loading methods. In the first, the cargo is loaded into the donor cell before the vesiculation process. While, in the second method, the cargo is loaded into the already produced vesicles. Created in Biorender.com, accessed on 9 January 2023.
6. Recent Development in Therapeutic Application of Red Blood Cell-Derived Extracellular Vesicles: From the Lab Side to the Industry Side
As discussed, RBCEVs possess several features that make them particularly suitable for therapeutic applications: (i) blood is easily accessible from blood banks, thus RBCs can be produced from blood at large scale and at low cost; (ii) autologous transfusion, from their own donor (or patient), or allogenic transfusion, from a universal 0-donor, of RBCEVs, are possible; (iii) RBCEVs are safer compared with other cell-derived EVs because they lack DNA, minimizing the risk of horizontal gene transfer; (iv) RBCEVs are completely nontoxic and have higher biocompatibility than synthetic EVs; (v) RBCEVs can be frozen and thawed many times without affecting their integrity and efficacy [18]. In this regard, a publication recently investigated different buffers and conditions to allow a longer stability of frozen EVs [55]. The authors demonstrated that EVs, resuspended in suitable buffers, can be stable for up to 2 years. Thus, RBCEVs can be developed into stable pharmaceutical products in the near future (Figure 3). This is probably the main feature that attracted the attention of several biotech and pharma companies.
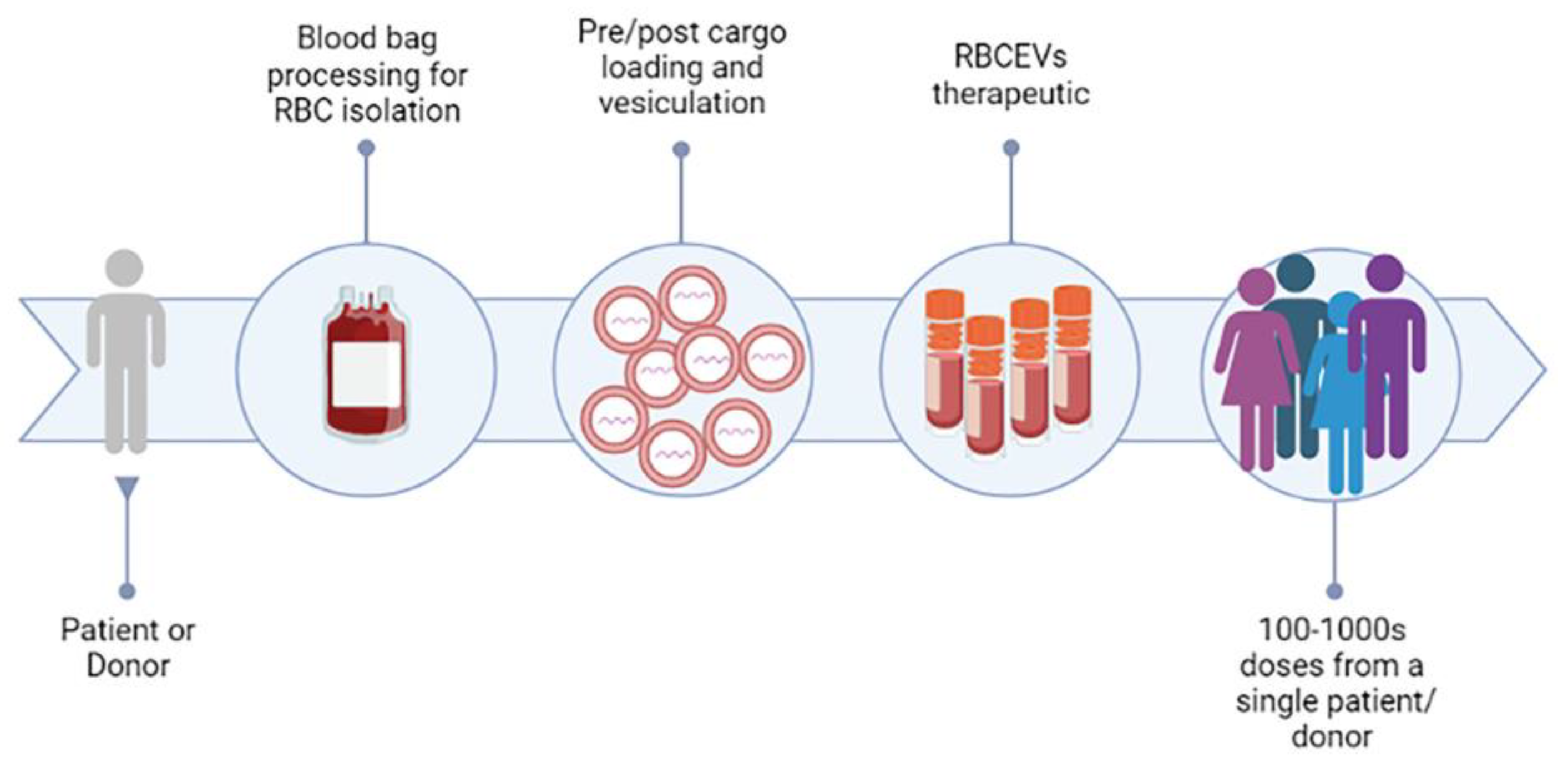
Figure 3. RBCEVs platform for clinical exploitation. The figure represents the road for the clinical exploitation of RBC-derived EVs, which could be achieved in the near future, and shows some of the advantages of this promising strategy. Created in Biorender.com, accessed on 14 December 2022.
6.1. From the Lab Side
In 2010, Chang and co-workers demonstrated the ability of RBCEVs to efficiently deliver ultra-small superparamagnetic iron oxide particles into human bone marrow mesenchymal stem cells for cellular magnetic resonance imaging in vitro and in vivo [56]. The novel method allowed for higher intracellular labeling efficiency and higher biosafety compared with traditional approaches. RBCEVs were shown for the first time to be biosafe and they can be used as potential delivery vehicles for clinical applications due to their autologous property; this research also gave rise to a patent that is cited in the next section.
But it was in 2018, that the most pioneering study was made by Usman and colleagues, where an efficient delivery system was developed for RNA-based therapeutics using RBCEVs [42]. Small and large RNAs, e.g., antisense oligonucleotides (ASOs), and large RNAs, such as mRNA, were electroporated into RBCEVs and transported to target cells in both solid and liquid tumors. Briefly, microRNA-125b-ASO-loaded RBCEVs significantly reduced both breast tumor growth by intratumoral injection and suppressed acute myeloid leukemia (AML) progression by systemic administration. In addition, genome-editing effects were also observed when RBCEVs were loaded with Cas9 mRNA and guide RNAs. The delivery efficiency was higher, and far less cytotoxicity was observed as compared to other commercial transfection reagents.
Moreover, exosome mimetics (EMs) were produced from red blood cells (RBCs), and the radiolabeling of the RBC-EMs for in vivo imaging was analyzed [45]. Engineered EMs from RBCs were produced on a large scale by a one-step extrusion method and further purified by density gradient centrifugation, the resulting RBC-EMs had a 130-fold greater yield compared to natural nanovesicles generated from RBCs and displayed enhanced in vivo biodistribution. RBC-EMs were labeled with technetium-99m (99mTc). The results demonstrated a simple yet large-scale production method for a novel type of RBC-EMs, which can be effectively labeled with 99mTc, and feasibly monitored in vivo by nuclear imaging. It shows that the RBC-EMs may be used as in vivo drug delivery vehicles [45].
In 2019, RBCEVs were applied in another study in which lipophilic drugs, such as camptothecin, were packaged within RBCEVs and administered to lung carcinoma cells, showing an improvement in targeted delivery when compared with synthetic lipid-based nanocarriers [57].
In 2020, Zhang and colleagues demonstrated that RBC-derived EVs loaded with miR-155 showed an excellent protective effect against acute liver failure, while those loaded with doxorubicin or sorafenib showed significant therapeutic effects against hepatocellular carcinoma without systemic toxicity in mice [58]. In the same year, other authors isolated RBCEVs from subjects infected by Plasmodium falciparum and loaded them with the antimalarial drugs atovaquone and tafenoquine [59]. They observed that the free drug was less effective than the RBCEV-loaded one, indicating that RBCEVs can potentially increase the efficacy of several small hydrophobic drugs used for the treatment of malaria.
In 2022, Jayasinghe and co-workers conjugated RBCEVs with several peptides and/or antibodies for targeted delivery of cargoes to cancer cells [60]. They conjugated RBCEVs with a cyclic peptide to specifically target CXCR4 or with a monoclonal antibody anti-CD33 to promote the specific binding and uptake of the conjugated EVs by leukemia cells expressing the corresponding receptors. CXCR4-conjugated RBCEVs were loaded with the pro-apoptotic peptide KLA, demonstrating that these were able to significantly suppress leukemia burden and increase survival in a leukemia xenografted mouse model. Antibody-conjugated RBCEVs were also used to deliver RNA antisense oligonucleotides to knock down FLT3 and miR-125b in cell lines and in patient-derived xenograft models of leukemia [60]. This research demonstrated for the first time that peptide/antibody-conjugated RBCEVs are biocompatible and non-immunogenic and can be used for targeted delivery of therapeutic peptides and RNAs for potential clinical applications. Finally, a novel nanocarrier composed of RBCEVs, surface-linked with doxorubicin using glutaraldehyde, was developed by [61]. The results demonstrated, once again, that drug-loaded RBCEVs could exert superior anticancer activity than free drug, both in vitro and in vivo.
In a very recent study, authors from the same group as Usman et al. [42] re-proposed RNA-loaded RBCEVs for potential immunotherapy [54]. In detail, they loaded the previous 3p-125b-ASO or a novel RIG-I agonist, namely an immunomodulatory RNA. The authors showed that the two agonists stimulated the RIG-I pathway and induced cell death in both mouse and human breast cancer cells. Significant suppression of tumor growth, coupled with increased immune cell infiltration mediated by the activation of the RIG-I cascade, was observed also in vivo after multiple intratumoral injections of RNA-loaded RBCEVs. Finally, they proposed also a targeted delivery using RBCEVs coupled with EGFR-binding nanobody, administrated intrapulmonary to mice, to facilitate the accumulation of RBCEVs in EGFR-positive breast cancer cells.
The main applications reported in the literature have been summarized in Table 1.
Table 1. Main findings on RBCs-based EVs as a drug delivery carrier.
| Reference | EV Production Method | Cargo-Loading Method | Cargo | Application | In Vitro | Pre-Clinical | Clinical |
|---|---|---|---|---|---|---|---|
| [56] | Chemical method, calcium chloride | Incubation under hypo-osmotic conditions | Ultrasmall superparamagnetic iron oxide (USPIO) particles | Magnetic resonance imaging | X | X | |
| [42] | Chemical method, calcium ionophore | Post-loading method, electroporation | Antisense oligonucleotides, Cas9 mRNA, and guide RNAs | Cancer therapy | X | X | |
| [45] | Physical method, extrusion | Post-loading method, incubation | Technetium-99m | In vivo imaging | X | ||
| [57] | Physical method, extrusion | Pre-loading method, hemolysis and incubation | Camptothecin and amphiphilic fluorophore | Cancer therapy | X | X | |
| [61] | Chemical method, calcium ionophore | Post-loading method, incubation | Doxorubicin | Cancer therapy | X | X | |
| [54] | Chemical method, calcium ionophore | Post-loading method, transfection and electroporation | Rig-I agonists, small RNAs | Cancer therapy | X | X | |
| [58] | Chemical method, calcium ionophore | Post-loading method, transfection | Antisense oligonucleotides, doxorubicin and sorafenib | Acute liver failure, cancer therapy | X | X | |
| [59] | Isolation of naturally produced RBCEVs | Post-loading method, incubation | Antimalarial drug, atovaquone and tafenoquine | Anti-malarial treatment | X | ||
| [60] | Chemical method, calcium ionophore | Post-loading method, transfection | Peptide, Antisense oligonucleotides, siRNA | Cancer therapy | X | X |
6.2. To the Industry Side
The first industrial exploitation of RBCEVs-like particles belongs to a researcher at the University of California. In particular, they produced RBC membrane-camouflaged nanoparticles by first producing RBC membrane-derived vesicles by hypotonic treatment and extrusion of RBCs, which were further combined with a polymeric nanoparticle core to produce RBC-derived nanoparticles (Patent Application Publication No. US 2013/0337066A1, Membrane encapsulated nanoparticles and method of use, 2013. Related patent documents: EP2714017 CN103857387 CA2873404 DK2714017 ES2685333 WO/2013/052167 EP3412282 JP2014518200 US20130337066). The inventive nanoparticle comprises (a) an inner core comprising a non-cellular material and (b) an outer surface comprising a cellular membrane derived from RBCs. These nanoparticles were tested in several applications, such as eliciting an immune response, and treating or preventing diseases or conditions, such as neoplasms or cancer, or diseases or conditions associated with cell membrane insertion of toxins. Later, Cellics Therapeutics invented another application of the aforementioned particles: a biomimetic toxin nanosponge that functions as a toxin decoy in vivo. These nanosponges absorb membrane-damaging toxins and can potentially treat a variety of injuries and diseases caused by pore-forming toxins (US 2017/0095510 A1, use of nanoparticles coated with red blood cell membranes to treat hemolytic diseases and disorders, 2017).
In 2019, Le et al. from the City University of Hong Kong proposed for the first time the use of native RBCEVs for gene therapy (US 2019/0054192 Isolation RBCEVs form RBCs for gene therapy, 2019. Related patent documents US20190054192 CN109402176). The invention comprises the purification and electroporation of the RBCEVs and applying the RNA-loaded EVs to target cells. Briefly, they proposed to stimulate the EVs’ production with calcium ionophore, followed by isolation by ultracentrifugation, and finally electroporation to load nucleic acids. Moreover, Minh Le and colleagues from the National University of Singapore and Cornell University developed a method of delivering nucleic acids to immune cells (e.g., T cells) using RBCEVs. These loaded immune cells can be used as immunotherapy to treat cancer (WO 2021/194425 A1, method of delivering nucleic acid to immune cells using RBCEVs, 2021. Related patent documents EP2021777014).
Some years later, researchers at the Imperial College in London proposed the use of targeted delivery of thrombolytic drugs to blood clots using RBCEVs platform WO 2021/123799 A1, red blood cell-derived vesicle, 2021. Related patent documents EP4076481). The invention also extends to a method of: (i) contacting a red blood cell with a hypotonic solution to produce a red blood cell ghost, and (ii) encapsulating an active agent using the red blood cell ghost to thereby produce a red blood cell-derived vesicle comprising an encapsulated active agent. Finally, step (iii) may further comprise extrusion through a filter at least once to produce EVs from ghost RBCs. Using this pre-loading method, RBCEVs have been encapsulated with thrombolytic drugs such as tissue plasminogen activator (tPA), which can further be used to treat ischemic strokes, myocardial infarction, and pulmonary embolism. RBCVs protect thrombolytic agents in the blood circulation, leading to improved stability and prolonged half-life, and temporarily suppress thrombolytic activity, leading to reduced hemorrhagic side effects.
In the same year, Carmine Therapeutics developed next-generation, non-viral based gene therapies to treat a wide range of diseases using RBCEVs (WO 2021/145821 A1, nucleic acid-loaded red blood cells extracellular vesicles 2021. Related patent documents WO/2021/145821 CA3164176 KR1020220127851 CN115151277 EP4090373 NZ789881 JP2022542905). The company proposed a method of isolating calcium ionophore-induced RBCEVs (as before) and further loading these vesicles with nucleic acids (DNA/RNA) by several methods comprising both electroporation and transfection. These platforms showed the ability to carry DNA or RNA payloads ranging from 20 bp to >30 kb and much more. Thus, by using RBCEVs as a drug delivery system, the limitations of viral-based gene therapies, such as immunogenicity, small payload capacity, and manufacturing challenges, can be overcome.
Finally, at the end of the year, Erytech Pharma also filed a patent application for the development of drug delivery RBCEVs from preloaded RBC (WO 2021/228832 A1, red blood cell extracellular vesicles (RCEVS) containing cargoes and methods of use and production thereof, 2021). First, red blood cells were loaded with the desired cargo by “hypotonic encapsulation” and then vesiculated by “strong agitation”. The cargo can be comprised of nucleic acids, proteins, small molecules, or components of a gene editing system, including CRISPR/Cas9. These loaded RBCEVs may be used to treat a variety of diseases and disorders, including autoimmune disorders, cancers, cardiovascular diseases, gastrointestinal diseases, genetic disorders, and inflammatory diseases.
To the best of our knowledge, these are the updates at the time of writing.
References
- Biagiotti, S.; Paoletti, M.F.; Fraternale, A.; Rossi, L.; Magnani, M. Drug delivery by red blood cells. IUBMB Life 2011, 63, 621–631.
- Rossi, L.; Fraternale, A.; Bianchi, M.; Magnani, M. Red Blood Cell Membrane Processing for Biomedical Applications. Front. Physiol. 2019, 10, 1070.
- Rossi, L.; Pierigè, F.; Aliano, M.P.; Magnani, M. Ongoing Developments and Clinical Progress in Drug-Loaded Red Blood Cell Technologies. BioDrugs 2020, 34, 265–272.
- Diez-Silva, M.; Dao, M.; Han, J.; Lim, C.T.; Suresh, S. Shape and biomechanics characteristics of human red blood cells in health and disease. MRS Bull. 2010, 35, 382–388.
- De Oliveira, S.; Saldanha, C. An overview about erythrocyte membrane. Clin. Hemorheol. Microcirc. 2010, 44, 63–74.
- Donadee, C.; Raat, N.J.H.; Kanias, T.; Tejero, J.; Lee, J.S.; Kelley, E.E.; Zhao, X.; Liu, C.; Reynolds, H.; Azarov, I.; et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 2011, 124, 465–476.
- Antonelou, M.H.; Seghatchian, J. Update on extracellular vesicles inside red blood cell storage units: Adjust the sails closer to the new wind. Transfus. Apher. Sci. 2016, 55, 92–104.
- Willekens, F.L.A.; Werre, J.M.; Groenen-Döpp, Y.A.M.; Roerdinkholder-Stoelwinder, B.; De Pauw, B.; Bosman, G.J.C.G.M. Erythrocyte vesiculation: A self-protective mechanism? Br. J. Haematol. 2008, 141, 549–556.
- Chiangjong, W.; Netsirisawan, P.; Hongeng, S.; Chutipongtanate, S. Red Blood Cell Extracellular Vesicle-Based Drug Delivery: Challenges and Opportunities. Front. Med. 2021, 8, 761362.
- Shah, M.D.; Bergeron, A.L.; Dong, J.F.; López, J.A. Flow cytometric measurement of microparticles: Pitfalls and protocol modifications. Platelets 2008, 19, 365–372.
- Duan, Z.Y.; Cai, G.Y.; Bu, R.; Lu, Y.; Hou, K.; Chen, X.M. Selection of urinary sediment miRNAs as specific biomarkers of IgA nephropathy. Sci. Rep. 2016, 6, 23498.
- Westerman, M.; Porter, J.B. Red blood cell-derived microparticles: An overview. Blood Cells. Mol. Dis. 2016, 59, 134–139.
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular vesicles from red blood cells and their evolving roles in health, coagulopathy and therapy. Int. J. Mol. Sci. 2021, 22, 153.
- Blanc, L.; De Gassart, A.; Géminard, C.; Bette-Bobillo, P.; Vidal, M. Exosome release by reticulocytes—An integral part of the red blood cell differentiation system. Blood Cells Mol. Dis. 2005, 35, 21–26.
- Kuo, W.P.; Tigges, J.C.; Toxavidis, V.; Ghiran, I. Red Blood Cells: A Source of Extracellular Vesicles. Methods Mol. Biol. 2017, 1660, 15–22.
- Zhang, D.X.; Kiomourtzis, T.; Lam, C.K.; Le, M.T.N. The Biology and Therapeutic Applications of Red Blood Cell Extracellular Vesicles. In Erythrocyte; IntechOpen: London, UK, 2019; ISBN 978-1-78984-210-4.
- Yang, L.; Huang, S.; Zhang, Z.; Liu, Z.; Zhang, L. Roles and Applications of Red Blood Cell-Derived Extracellular Vesicles in Health and Diseases. Int. J. Mol. Sci. 2022, 23, 5927.
- Khalyfa, A.; Sanz-Rubio, D. The mystery of red blood cells extracellular vesicles in sleep apnea with metabolic dysfunction. Int. J. Mol. Sci. 2021, 22, 4301.
- Burger, P.; Hilarius-Stokman, P.; De Korte, D.; Van Den Berg, T.K.; Van Bruggen, R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 2012, 119, 5512–5521.
- Doss, J.F.; Corcoran, D.L.; Jima, D.D.; Telen, M.J.; Dave, S.S.; Chi, J.T. A comprehensive joint analysis of the long and short RNA transcriptomes of human erythrocytes. BMC Genom. 2015, 16, 952.
- Huang, H.; Zhu, J.; Fan, L.; Lin, Q.; Fu, D.; Wei, B.; Wei, S. MicroRNA Profiling of Exosomes Derived from Red Blood Cell Units: Implications in Transfusion-Related Immunomodulation. Biomed Res. Int. 2019, 2019, 2045915.
- Sun, L.; Yu, Y.; Niu, B.; Wang, D. Red Blood Cells as Potential Repositories of MicroRNAs in the Circulatory System. Front. Genet. 2020, 11, 442.
- Vidal, M. Exosomes in erythropoiesis. Transfus. Clin. Biol. 2010, 17, 131–137.
- Alaarg, A.; Schiffelers, R.M.; Van Solinge, W.W.; Van Wijk, R. Red blood cell vesiculation in hereditary hemolytic anemia. Front. Physiol. 2013, 4, 365.
- Tissot, J.D.; Canellini, G.; Rubin, O.; Angelillo-Scherrer, A.; Delobel, J.; Prudent, M.; Lion, N. Blood microvesicles: From proteomics to physiology. Transl. Proteom. 2013, 1, 38–52.
- Harisa, G.I.; Badran, M.M.; Alanazi, F.K. Erythrocyte nanovesicles: Biogenesis, biological roles and therapeutic approach: Erythrocyte nanovesicles. Saudi Pharm. J. 2017, 25, 8–17.
- Liu, C.; Zhao, W.; Christ, G.J.; Gladwin, M.T.; Kim-Shapiro, D.B. Nitric oxide scavenging by red cell microparticles. Free Radic. Biol. Med. 2013, 65, 1164–1173.
- Danesh, A.; Inglis, H.C.; Jackman, R.P.; Wu, S.; Deng, X.; Muench, M.O.; Heitman, J.W.; Norris, P.J. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood 2014, 123, 687–696.
- Fischer, D.; Büssow, J.; Meybohm, P.; Weber, C.F.; Zacharowski, K.; Urbschat, A.; Müller, M.M.; Jennewein, C. Microparticles from stored red blood cells enhance procoagulant and proinflammatory activity. Transfusion 2017, 57, 2701–2711.
- Wannez, A.; Devalet, B.; Chatelain, B.; Chatelain, C.; Dogné, J.M.; Mullier, F. Extracellular Vesicles in Red Blood Cell Concentrates: An Overview. Transfus. Med. Rev. 2019, 33, 125–130.
- Koshiar, R.L.; Somajo, S.; Norström, E.; Dahlbäck, B. Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulation. PLoS ONE 2014, 9, e104200.
- Levin, G.; Sukhareva, E.; Lavrentieva, A. Impact of microparticles derived from erythrocytes on fibrinolysis. J. Thromb. Thrombolysis 2016, 41, 452–458.
- Buttari, B.; Profumo, E.; Riganò, R. Crosstalk between red blood cells and the immune system and its impact on atherosclerosis. Biomed Res. Int. 2015, 2015, 616834.
- Valkov, N.; Das, A.; Tucker, N.R.; Li, G.; Salvador, A.M.; Chaffin, M.D.; De Oliveira, G.P.; Kur, I.; Gokulnath, P.; Ziegler, O.; et al. SnRNA sequencing defines signaling by RBC-derived extracellular vesicles in the murine heart. Life Sci. Alliance 2021, 4, e202101048.
- Regev-Rudzki, N.; Wilson, D.W.; Carvalho, T.G.; Sisquella, X.; Coleman, B.M.; Rug, M.; Bursac, D.; Angrisano, F.; Gee, M.; Hill, A.F.; et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 2013, 153, 1120–1133.
- Ataga, K.I. Hypercoagulability and thrombotic complications in hemolytic anemias. Haematologica 2009, 94, 1481–1484.
- Aharon, A.; Rebibo-Sabbah, A.; Tzoran, I.; Levin, C. Extracellular Vesicles in Hematological Disorders. Rambam Maimonides Med. J. 2014, 5, e0032.
- Herring, J.M.; Mcmichael, M.A.; Smith, S.A. Microparticles in health and disease. J. Vet. Intern. Med. 2013, 27, 1020–1033.
- Vázquez-Ríos, A.J.; Molina-Crespo, Á.; Bouzo, B.L.; López-López, R.; Moreno-Bueno, G.; de la Fuente, M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J. Nanobiotechnol. 2019, 17, 85.
- Sakai-Kato, K.; Yoshida, K.; Takechi-Haraya, Y.; Izutsu, K.I. Physicochemical Characterization of Liposomes That Mimic the Lipid Composition of Exosomes for Effective Intracellular Trafficking. Langmuir 2020, 36, 12735–12744.
- Lai, R.C.; Yeo, R.W.Y.; Tan, K.H.; Lim, S.K. Exosomes for drug delivery—A novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013, 31, 543–551.
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018, 9, 2359.
- Sudnitsyna, J.; Skverchinskaya, E.; Dobrylko, I.; Nikitina, E.; Gambaryan, S.; Mindukshev, I. Microvesicle Formation Induced by Oxidative Stress in Human Erythrocytes. Antioxidants 2020, 9, 929.
- Prudent, M.; Crettaz, D.; Delobel, J.; Seghatchian, J.; Tissot, J.D.; Lion, N. Differences between calcium-stimulated and storage-induced erythrocyte-derived microvesicles. Transfus. Apher. Sci. 2015, 53, 153–158.
- Gangadaran, P.; Hong, C.M.; Oh, J.M.; Rajendran, R.L.; Kalimuthu, S.; Son, S.H.; Gopal, A.; Zhu, L.; Baek, S.H.; Jeong, S.Y.; et al. In vivo Non-invasive Imaging of Radio-Labeled Exosome-Mimetics Derived From Red Blood Cells in Mice. Front. Pharmacol. 2018, 9, 817.
- Sangha, G.S.; Weber, C.M.; Sapp, R.M.; Setua, S.; Thangaraju, K.; Pettebone, M.; Doctor, A.; Buehler, P.W.; Clyne, A.M. Mechanical Stimuli such as Shear Stress and Piezo1 Stimulation Generate Red Blood Cell Extracellular Vesicles. bioRxiv 2022, 21, 7885.
- Han, Y.; Jones, T.W.; Dutta, S.; Zhu, Y.; Wang, X.; Narayanan, S.P.; Fagan, S.C.; Zhang, D. Overview and update on methods for cargo loading into extracellular vesicles. Processes 2021, 9, 356.
- Kanada, M.; Bachmann, M.H.; Hardy, J.W.; Frimannson, D.O.; Bronsart, L.; Wang, A.; Sylvester, M.D.; Schmidt, T.L.; Kaspar, R.L.; Butte, M.J.; et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl. Acad. Sci. USA 2015, 112, E1433–E1442.
- Familtseva, A.; Jeremic, N.; Tyagi, S.C. Exosomes: Cell-created drug delivery systems. Mol. Cell. Biochem. 2019, 459, 1–6.
- Rossi, L.; Serafini, S.; Magnani, M. Red Blood Cell Loading: A Selection of Procedures. In Erythrocyte Engineering for Drug Delivery and Targeting; Bioscience, K.A.L., Ed.; Georgetown: New York, NY, USA, 2003; pp. 1–18.
- Magnani, M.; Serafini, S.; Fraternale, A.; Antonelli, A.; Biagiotti, S.; Pierigè, F.; Sfara, C.; Rossi, L. Red blood cell-based delivery of drugs and nanomaterials for therapeutic and diagnostic applications. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valenciaz, CA, USA, 2011; pp. 309–354. ISBN 1588831876.
- Baker, R.F. Entry of ferritin into human red cells during hypotonic haemolysis. Nature 1967, 215, 424–425.
- Baker, R.F.; Gillis, N.R. Osmotic hemolysis of chemically modified red blood cells. Blood 1969, 33, 170–178.
- Peng, B.; Nguyen, T.M.; Jayasinghe, M.K.; Gao, C.; Pham, T.T.; Vu, L.T.; Yeo, E.Y.M.; Yap, G.; Wang, L.; Goh, B.C.; et al. Robust delivery of RIG-I agonists using extracellular vesicles for anti-cancer immunotherapy. J. Extracell. Vesicles 2022, 11, e12187.
- Görgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles 2022, 11, e12238.
- Chang, M.; Hsiao, J.K.; Yao, M.; Chien, L.Y.; Hsu, S.C.; Ko, B.S.; Chen, S.T.; Liu, H.M.; Chen, Y.C.; Yang, C.S.; et al. Homologous RBC-derived vesicles as ultrasmall carriers of iron oxide for magnetic resonance imaging of stem cells. Nanotechnology 2010, 21, 235103.
- Malhotra, S.; Dumoga, S.; Sirohi, P.; Singh, N. Red Blood Cells-Derived Vesicles for Delivery of Lipophilic Drug Camptothecin. ACS Appl. Mater. Interfaces 2019, 11, 22141–22151.
- Zhang, G.; Huang, X.; Xiu, H.; Sun, Y.; Chen, J.; Cheng, G.; Song, Z.; Peng, Y.; Shen, Y.; Wang, J.; et al. Extracellular vesicles: Natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. J. Extracell. Vesicles 2020, 10, e12030.
- Borgheti-Cardoso, L.N.; Kooijmans, S.A.A.; Chamorro, L.G.; Biosca, A.; Lantero, E.; Ramírez, M.; Avalos-Padilla, Y.; Crespo, I.; Fernández, I.; Fernandez-Becerra, C.; et al. Extracellular vesicles derived from Plasmodium-infected and non-infected red blood cells as targeted drug delivery vehicles. Int. J. Pharm. 2020, 587, 119627.
- Jayasinghe, M.K.; Pirisinu, M.; Yang, Y.; Peng, B.; Pham, T.T.; Yu Lee, C.; Tan, M.; Vu, L.T.; Dang, X.T.T.; Pham, T.C.; et al. Surface-engineered extracellular vesicles for targeted delivery of therapeutic RNAs and peptides for cancer therapy. Theranostics 2022, 12, 3288–3315.
- Wu, S.-H.H.; Hsieh, C.-C.C.; Hsu, S.-C.C.; Yao, M.; Hsiao, J.-K.K.; Wang, S.-W.W.; Lin, C.-P.P.; Huang, D.-M.M. RBC-derived vesicles as a systemic delivery system of doxorubicin for lysosomal-mitochondrial axis-improved cancer therapy. J. Adv. Res. 2020, 30, 185–196.
More
