Potato (Solanum tuberosum L.) is a valuable food crop with great importance in ensuring food security worldwide. One of the most acute problems of modern agriculture and food industry is the loss of potato tubers (about 40-60% of the total harvest) during storage from diseases. Beneficial antagonistic bacteria Bacillus subtilis, generally recognized as safe microorganisms (GRAS) to use in the food industry, are considered a bio‐active and eco‐friendly agent for controlling postharvest decays of potato. Of special interests are endophytic B. subtilis, living inside plant tissues, which allows them to be less dependent on external environmental factors (compared to rhizosphere and phyllosphere strains) while exhibiting "useful" features. Due to it is difficult to select an individual effective microbial strain with a broad spectrum of activity against a range of pathogens an interest is co-application of B. subtilis with other methods (biological, physical) in an integrated vision of disease management. In this study, the effect of endophytic B. subtilis (strains 10‐4, 26D) and their compositions with salicylic acid (SA) on some resistance and quality traits of stored potatoes infected with Fusarium oxysporum-caused dry rot were studied. The results that are presented here establish that the treatment of potato tubers immediately before storage with endophytic bacteria B. subtilis (10‐4, 26D) individually and in combinations with SA reduced the incidence of F. oxysporum‐mediated dry rot (up to 50%) in potatoes during long‐term storage, with the highest protective effect upon application of composition B. subtilis 10‐4 + SA.
- Endophytic Bacillus subtilis
- Salicylic Acid
- Postharvest Dacays of Potato
- Resistance
- Biotechnology
1. Supression of F. Oxysporum Development in Stored Potato Tubers by Endophytic B. Subtilis (10-4, 26D) and B. Subtilis (10-4, 26D) + SA
The artificial infection of potato tubers by F. oxysporum overtime led to a gradual increase in symptoms of Fusarium dry rot, reaching 100% by six months of storage was found (Figure 1A,B). The bacterization of tubers immediately before storage with pre-established [1] concentrations of B. subtilis (10-4, 26D), in compositions with and without SA, resulted in the reduced intensity of F. oxysporum development, manifested as a 30–50% decrease in the area of lesions after long-time storage (Figure 1A,B). The most positive effect in the suppression of F. oxysporum development in stored tubers treated with 10-4 + SA was observed.
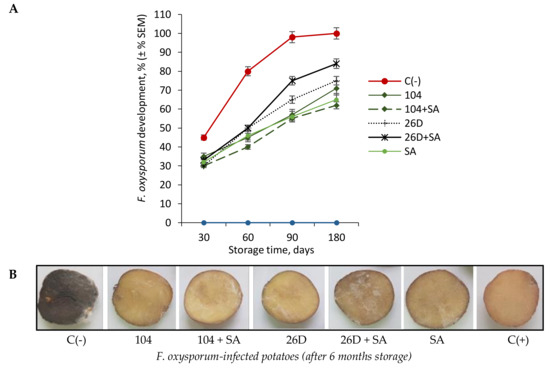
Figure 1. Effect of B. subtilis 10-4 (104), B. subtilis 26D (26D), B. subtilis 10-4 + salicylic acid (SA) (104 + SA), and B. subtilis 26D + SA (26D + SA) on F. oxysporum development in potatoes during long-time storage for six months (A) and pictures of tubers stored six months after infestation with F. oxysporum and coated with B. subtilis strains 10-4, 26D, and their compositions with SA (B). For each treatment was used 30 mini-tubers in three replicates (± SEM). C(-)—negative control tubers infected before storage with F. oxysporum; 104—tubers infected with F. oxysporum and treated with B. subtilis 10-4; 104 + SA—tubers infected with F. oxysporum and treated with composition B. subtilis 10-4 + SA; 26D—tubers infected with F. oxysporum and treated with B. subtilis 26D; B. subtilis 26D + SA—tubers infected with F. oxysporum and treated with B. subtilis 26D + SA, SA—tubers infected before storage with F. oxysporum and treated with SA; C(+)—positive control tubers without infection and treatments.
2. Vitro Studies
In vitro studies also showed that B. subtilis 10-4 and 26D have antagonistic activity against the phytopathogenic fungus F. oxysporum (Figure 2A). The microscopic observation of the F. oxysporum fungal mycelia clearly revealed morphological variations. The structure of the F. oxysporum mycelia was well organized in the absence of the bacterial culture medium (Figure 2B), while numerous gaps of mycelia appeared and macroconidia were produced in the presence of the culture medium of strains 10-4 and 26D (Figure 2B).
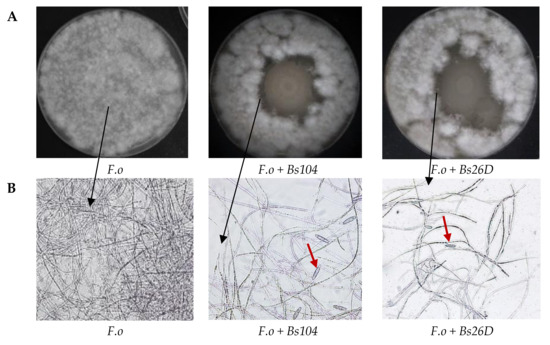
Figure 2. In vitro antagonistic activity of tested B. subtilis 10-4 (Bs104) and B. subtilis 26D (Bs26D) against the phytopathogenic fungus F. oxysporum (F.o) (A) and microscopic visualizations of the F. oxysporum fungal growth and morphology in the absence and presence of B. subtilis 10-4 and 26D (B). The observation was done using a scanning electron microscope Biozero BZ-8100E (Keyence Co., Osaka, Japan). F.o—F. oxysporum; Bs—B. subtilis. Red arrows mean macroconidia produced by F. oxysporum.
References
- Lastochkina, O.; Baymiev, A.; Shayahmetova, A.; Garshina, D.; Koryakov, I.; Shpirnaya, I.; Pusenkova, L.; Mardanshin, I.; Kasnak, C.; Palamutoglu, R. Effects of endophytic Bacillus subtilis and salicylic acid on postharvest diseases (Phytophthora infestans, Fusarium oxysporum) development in stored potato tubers. Plants 2020, 9, 76.
