Elaeodendron is a genus of tiny trees, shrubs, vines, and herbs consisting of about 23 species. It is used in traditional medicine and has a wide range of pharmacological activities. From the plants in this genus, flavonoids, terpenoids, cardiac glycosides, and cardenolides have been isolated. Preclinical investigations have also revealed antiviral, anti-HIV, anticancer, antiproliferative, antioxidant, antifungal, anti-inflammation, cytotoxic, anti-plasmodial, anti-arthritic, antibacterial, and anti-diabetic activities. Bioactive substances found in Elaedendron that function in a variety of ways are related to these biological processes.
- Elaeodendron
- Celastraceae
- cardenolides
1. Introduction
2. Traditional Uses
Elaeodendron buchananii (Loes.) Loes. is an evergreen shrub or tree with a branching, rounded crown found in eastern Africa, particularly Uganda and Kenya [9]. Despite its toxic nature, E. buchananii is occasionally utilized in conventional practice of medicine. Leaf extracts are used to treat fever, as an abortifacient, oxytocic, tonic, and vermifuge [10][11][12]. Chewing the leaves is considered beneficial for the treatment of diarrhea. Gastrointestinal problems, bloody coughing, excessive uterine bleeding, and infertility are treated using root decoctions. Syphilis is treated using root powder [10][13][14]. On wounds, the root powder is administered topically. The bark decoction is also used to cure leukemia [9]. Elaeodendron croceum (Thunb.) DC., also known as saffron, saffron wood, and forest saffron, is an evergreen tree with a tidy, vertical frame found in various parts of South Africa (Ladismith, KwaZulu-Natal, Limpopo, Southern Cape forests) and in Zimbabwe (Mount Cherinda) [4]. The bark of this plant is used as a febrifuge and emetic in therapeutic approaches to treat opportunistic infections caused by the human immunodeficiency virus (HIV) [4][15]. Tuberculosis and other associated disorders, such as blood in sputum, chest congestion, cough, and sore throat have historically been treated and managed using the bark [15]. The roots, bark, and leaves of the plant are used as herbal treatments to clear the gastrointestinal system and control fever [16]. Preparations of Elaeodendron glacum (Rottb.) Pers. have been employed by conventional healers as a remedy for a number of diseases such as diabetes. As sternutatories, the dried and powdered leaves are employed [17]. The dried leaves are also burned, and the resulting smoke is utilized as a disinfectant to treat some nerve illnesses, especially to rouse women from hysterics [18]. Headache is relieved by snuffing the powdered leaves. Fresh root bark is ground into a paste with water and applied to swellings as a poultice. The root is reported to have anti-snake-venom properties. As an emetic, cold-water infusion of the pulverized roots is employed [1][19]. Elaeodendron orientale Jacq., sometimes known as the fake olive, is an indigenous plant of the Mascarene Islands and Madagascar [6]. The bark has traditionally been used to cure chest infections, venereal illness, and scorpion fish poisoning. The leaves are emetic and astringent. The combination of leaves with those of Kalanchoe pinnata (Crassulaceae) generate bufadienolides, used to alleviate hypertension and treat seafood allergies [6].3. Bioactive Compounds from Elaeodendron Species
Bioactive chemicals are extra nutritional components detected in tiny concentrations in plants and foods that provide health advantages in addition to the basic nutritional value [20]. Bioactive substances appear to have significant immunological, behavioural, and physiological effects. They are being examined extensively to determine their effect on the human body. They are gaining popularity in various fields, including contemporary pharmacology, food business, plant science, nanobioscience, cosmetics, and agrochemicals [20]. Plant bioactive chemicals are categorized using a variety of criteria. Strongly linked species of plants typically generate similar or slightly structurally comparable active compounds. It might be helpful to categorize active molecules based on the genera and families in which they exist. However, there are several situations when genetically unrelated organisms create identical secondary chemicals. The bioactive chemical compounds are the major emphasis. Thus, it is helpful to organize them into biochemical and chemical classes [21]. Elaeodendron species are rich in various biologically active chemicals responsible for a wide range of pharmacological actions. Environmental circumstances, climatic conditions, harvesting season and methods, genetic conditions, species variety, plant part and age, vegetative phase, and soil may all influence the quantitative and qualitative composition of bioactive chemicals [14][22]. Table 1 lists the chemical compounds found in Elaeodendron species (Figure 1).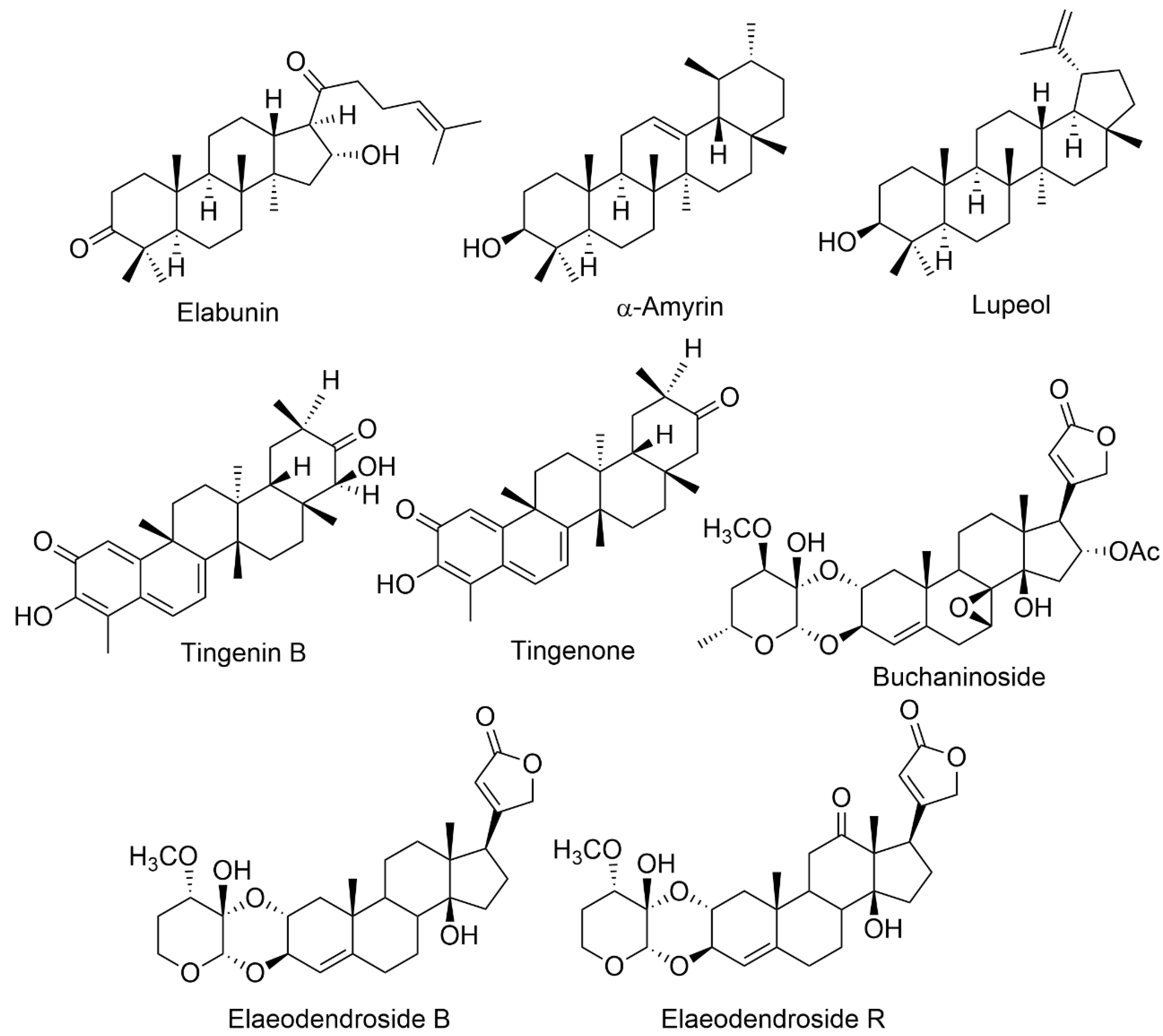
| Species | Isolated Compounds | Traditional Uses | Part Used | Reported Biological Activity | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. buchananii | Elabunin; lupeol; 19α, 28-trihydroxyurs-12- en-23-oic acid; 3β, 11α, 3β-acetoxy-19α, 23, 28-trihydroxyurs-12-ene; 3-oxo-19α, buchaninoside; 19α-trihydroxyurs-12-en-23, 28-dioic acid; mutangin; methyl 3β-acetoxy-11α, 28 dihydroxyurs-12-en-24-oic acid | Fever, diarrhea gastrointestinal problems, bloody coughing, excessive uterine bleeding, infertility, syphilis, wounds, and leukemia | Leaves, Roots Bark | Anticancer, gastrointestinal disturbances, antimicrobial | [9][11] | ||||||
| E. croceum | 30-Hydroxylup 20(29)-en-3-one; (+)-6R,13R-11,11- dimethyl-1,3,8,10-tetrahydroxy-9-methoxy-peltogynan; galactitol; canophyllol; (−)-4′-0-methoxyepigallocatechin; tingenin B; ouratea-proanthocyanidin A; tingenone; 3-hydroxylupeol; 11α-hydroxy-β-amyrin; naringenin | Tuberculosis, blood in sputum, chest congestion, cough, sore throat, gastrointestinal system, fever | Stem bark | Anti-HIV, antibacterial, anti-arthritic, antimycobacterial, antifungal, antioxidant, anti-inflammatory, cytotoxic | [4][15] | ||||||
| E. glacum | 30-Hydroxylup-20(29)-en-3-one; tingenone; canophyllol; tingenin B; 3-hydroxylupeol; elaeodendroside; isocardenolide | Diabetes, sternutatories, nerve illnesses, swellings, headaches, emetic | Leaves, Root bark | Ani-diabetic, anti-snake-bite properties | [17] | ||||||
| E. orientale | Elaeodendroside F; elaeodendroside G; elaeodendroside T; elaeodendroside B; elaeodendroside C; elaeodendroside R; 20(22)-dienolid,6β,8β,11α,14β-tetrahydroxy-12-oxo-2α-O; 11α,14β-dihydroxy-2α-O;3β- O-(30α-methoxy-40-deoxy-50-dehydroxymethyl hexosulose)-card-4; 20(22)-dienolide, 3β-O-(20α,30β-methylendioxy)-40-desoxy-50-deshydroxymethyl-hexosu- lose]-card-4, 11α,14β-dihydroxy-2α-O; 3β-O-(30 α-methoxy-40-deoxy-50-dehydr-oxymethyl-hexosulose)-card-4; 20(22)-dienolide | chest infections, venereal illness, scorpion fish poisoning, astringent emetic, hypertension | Leaves, root bark | Anti-arthritic, antiproliferative, anticancer | [3][6] | ||||||
| E. schweinfurthianum | 3α-Hydroxyfriedelane; α-amyrin acetate; α-amyrin; 3-oxo-29-hydroxyfriedelane; β-sitosterol; lanosterol; stigmasterol; 3-oxofriedelane; 3-oxofriedelan-28-al | Fever | Roots | Antibacterial, anti-HIV, anti-plasmodial | [1] | ||||||
| E. schlechteranum | 4′,4″-Di-O-methyl-prodelphinidin; B | 4, | 3β,29-dihydroxyglutin-5-ene; 4′- | O | -methyl-epigallocatechin; tingenin B; 4′- | O | -methylgallocatechin; cangoronine methyl ester | Menstrual irregularities, anaemia, heart issues, high blood pressure, basic body discomfort, inflammatory disease, carbuncles boils, wounds | Roots, stem bark, root bark, leaf | Anti-HIV, anti-inflammatory | [23] |
| E. transvaalense | 4′- | O | -Methyl-epigallocatechin; canophyllal; (+)-, 11,11-dimethyl-1,3,8,10-trahydroxy-9-methoxypeltogynan; 6β-hydroxy-lup-20(30)-en-3-one; galactitol; hydroxylup-20(29)-ene-3-one; lup-20(29)-ene-30-hydroxy-3-one; Ψ-taraxastanonol; lup-20(30)-ene-3α,29-diol; lup-20(30)-ene-3α,29-diol; β-sitosterol; 3,28-dihydroxylbetuli-20(29)-ene; lup-20(30)-ene-3α,29-diollup-20(29)-ene-30-hydroxy-3-one; 4′-O-methyl-epigallocatechin; 3-oxo-28-hydroxylbetuli-20(29)-ene; 30-hydroxylup-20(29)- ene-3-one. | Diarrhea, stomachache, rashes, skin infections, inflammations, menorrhagia, women’s fertility issues, hypertension, HIV, sexually transmitted diseases (STDs). | Root bark | Anti-HIV, anti-inflammatory, antimicrobial, antioxidant, antimalarial, cytotoxic | [7][24][25] | ||||
| E. xylocarpum | 3,25-Epoxy-olean-12-ene; 3β,21a-dihydroxyglut-5-ene; baruol; friedelin; cangoronine; cangoronine methyl ester; glutinol; 3β,29-dihydroxyglut-5-ene; wilforol E; 6β,30-dihydroxylup-20(29)-en-3-one; 6β-hydroxy-3-oxolup-20(29)-en-30-al; 3-oxolup-20(29)-en-30-oic acid; 3β,6β,20-trihydroxylupane; 11α,28-dihydroxylup-20(29)-en-3-one; 3- oxolup-20(29)-en-30-al; ochraceolide A; 12 3-oxo-30 hydroxylupane; 11 3-epiglochidiol; lupenone; botulin; 11 6β,20-dihydroxylupan-3-one; 16 lupan-3β-caffeate; 11 betulin-3β-caffeate; glochidiol; 3-epibetulin; betulone; 11α hydroxyglochidone; lupeol; rigidenol; nepeticin; glochidone; 25-hydroxylupeol; 15 3β,30-dihydroxylupane; 3-epinepeticin; 3b,29-Dihydroxy-olean-18-ene; 29-Hydroxy-3-oxo-olean-18-ene; 6b,29-Dihydroxy-3-oxo-olean-18-ene; 6b-Hydroxy-3-oxo-olean-18-ene; 3b,21a-Dihidroxy-olean-18-ene; 3b,6b-Dihidroxy-olean-18-ene; 21a-Hydroxy-3-oxo-olean-18-ene; 3b,11a,28-Trihydroxy-olean-18-ene; 29-Acetoxy-3-oxo-olean-18-ene; 3b,21a-Diacetoxy-olean-18-ene; 3b-Acetoxy-6b-hydroxy-olean-18-ene; 6β,30-Dihydroxylup-20(29)-en-3-one; 6β-Hydroxy-3-oxolup-20(29)-en-30-al; 3-Oxolup-20(29)-en-30-oic acid; 3β,6β,20-Trihydroxylupane; 1β,3α,28-Trihydroxylup-20(29)-ene; 11α,28-Dihydroxy-3-oxolup-20(29)-ene; 3β,28-Di-O-octanoylbetulin; 28-O-(1-Naphthoyl)botulin; 3β,28-Di-O-(1-naphthoyl)botulin; 28-Oacetyl-3β,20,29-trihydroxylupane; 28-O-acetyl20R,29-epoxy-3β-hydroxylupane; 2 (28-O-acetyl-3β-hydroxylup-20(29)-en30-al; 3β,30-di-O-acetyllup-20(29)-ene; 2-bromo-3-oxolup-20(29)-ene;11α-O-acetyl-3-oxolup-20(29)-ene;11α-O-Acetyl-30-chloro-3-oxolup-1,20(29)-diene | Stimulant | Root bark | Anti-HIV | [26][27] |
4. Pharmacological Properties of Elaeodendron Species
4.1. Antioxidant Activity
4.2. Anti-inflammatory Activity
Odeyemi and Afolayan used the protein denaturation test using diclofenac as a control sample to assess the anti-inflammatory effects of E. croceum stem bark and leaf acetone extracts. The extracts indicated activity, with IC50 values of 0.9 mg/mL and 1.9 mg/mL for leaf and stem bark extracts, respectively, whereas diclofenac (positive control) had an IC50 value of 0.3 mg/mL. The extracts had a considerable inhibitory effect on protein denaturation, indicating that they may have anti-inflammatory properties [28]. Olaokun et al. examined the inhibitory effects of E. croceum bark acetone extracts on the pro-inflammatory enzyme 5-lipoxygenase (5-LOX) using HeLa cervix carcinoma cells and human pancreatic cancer cell lines Panc-1 and Capan-2 as the cell lines and using quercetin as the control sample to investigate if the extract reduce inflammation. The extract reduced 5-lipoxygenase activity with an IC50 of 75.5 g/mL [29]. Elisha et al. examined the anti-inflammatory properties of E. croceum leaves acetone extract by measuring nitric oxide (NO) generation suppression and 15-lipoxygenase enzyme inhibition in lipopolysaccharide (LPS) activated RAW 264.7 macrophages. The preparations decreased NO generation in LPS-stimulated RAW 264.7 macrophage in a dose dependent manner. The extract E. croceum (IC50 = 26.2 µg/mL) demonstrated significant activity against 15-lipoxygenase activity than that of the control sample quercetin IC50 of 53.7 µg/mL [30].4.3. Antibacterial Activity
Khumalo et al. examined the antimicrobial property of E. transvaalense stem bark extracts and components in methanol and dichloromethane. 6β-hydroxy-lup-20(29)-ene-3-one,4′-O-methylepigallocatechin, lup-20(30)-ene-3α,29-diol, and 30-hydroxylup-20(29)-ene-3-one were tested against Salmonella typhimurium, Staphylococcus epidermidis [31] Staphylococcus aureus, Escherichia coli, Shigella sonnei, and Pseudomonas aeruginosa using a micro-titer plate broth two-fold serial dilution experiment with ciprofloxacin as the control sample. The extract and compounds had moderate antibacterial activity, with minimum inhibitory concentration values 0.1 mg/mL to 1.7 mg/mL. Using the serial broth microdilution assay and ciprofloxacin as a positive control. Mamba et al. investigated the antimicrobial activities of E. transvaalense bark ethanol extracts and the molecules 4′-O-methyl-epigallocatechin, lup-20(30)-ene-3,29-diol and lup-20(29)-ene-30-hydroxy-3-one isolated from the plant against Neisseria gonorrhoeae, Oligella ureolytica, and Gardnerella vaginalis. MIC values for the extracts and compounds varied from 1.6 mg/mL to 12.5 mg/mL, whereas the positive control had a MIC of 0.01 mg/mL [16]. McGaw et al. used disc-diffusion and micro-dilution assays to test the antimicrobial activity of E. transvaalense bark aqueous and hexane ethanol extracts against Staphylococcus aureus, Klebsiella pneumoniae, Bacillus subtilis, and Escherichia coli with neomycin as a positive control. Water and ethanolic extracts were potent against Bacillus subtilis and Staphylococcus aureus, with MICs ranging from 0.1 mg/mL to 0.8 mg/mL [32]. Using the agar dilution method, Tshikalanga et al. investigated the antimicrobial activities of E. transvaalense chloroform and aqueous bark extracts against Enterobacter cloacae, Enterobacter aerogenes, Bacillus pumilus, Bacillus cereus, Klebsiella pneumoniae, Bacillus subtilis, and Escherichia coli. The extracts had MIC values between 20 mg/mL to 50 mg/mL against Bacillus cereus, Bacillus pumilus, Bacillus subtilis, and Staphylococcus aureus [7].
4.4. Cytotoxic Activity and Antiproliferative Activity
Prinsloo et al. used 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay on Vero cells to assess the cytotoxicity of semi-purified E. croceum stem bark extracts. On cell lines with a treatment response of 250 (therapeutic index), the extract demonstrated only 20% toxicity at a concentration of 25 μg/mL [33]. Using the MTT assay on Vero cells, Elisha et al. assessed the cytotoxic effects of an acetone extract of E. croceum leaves. The extract revealed activity with an LC50 of 5.2 μg/mL and SI (selectivity index) values between 0.01 to 0.07, whereas the standard drug, doxorubicin, had an LC50 of 8.3 μg/mL [34][35].4.5. Anti-fungal Activity
Mamba et al. used the broth microdilution technique to test the antifungal activity of E. croceum ethanol bark extract using ciprofloxacin as the control sample against Candida albicans. The extract showed activity with a minimum inhibitory concentration (MIC) of 1.6 mg/mL, whereas ciprofloxacin had an MIC of <0.01 mg/mL [16]. The antifungal properties of E. croceum extracts demonstrate the species’ potential as an herbal remedy against fungal and microbial diseases.4.6. Anti-HIV Activity
Prinsloo et al. tested the anti-HIV effects of E. croceum stem bark by measuring signaling pathway inhibition in the MT-2 VSV-pseudotyped and HeLa-TAT-Luc recombinant virus tests. At 100 ng/mL, the extracts inhibited signaling pathways effectively [36]. Mamba et al. used an RT (non-radioactive HIV-reverse transcriptase) colorimetric test with doxorubicin as a standard drug to assess the anti-HIV activity of E. croceum ethanol bark extract against recombinant HIV-1 enzyme. The extract had a lower inhibitory activity of 30.2%, whereas doxorubicin, a positive control, had a 96.5% inhibitory activity [16]. The anti-HIV effects of E. croceum extracts and the substance digitoxigenin-glucoside identified from the plant support the plants’ traditional use in South Africa to treat HIV opportunistic infections [16]. E. schlechteranum 80% MeOH extract and 4-O-Methylgallocatechin-(48)-4-O-methylepigallocatechin isolated from the extract were evaluated against HIV-1 (strain IIIB) and HIV-2 (strain ROD) [37][38]. The anti-HIV testing and cytotoxicity evaluation of the fractions in MT-4 cells (expressing HTLV-1 Tax and permissive for replication of an HIV-1 gp41 mutant lacking the cytoplasmic tail) revealed that polyphenolic chemicals are responsible for at least some of the anti-HIV-1 action. HIV-1 reverse transcriptase and HIV-1 integrase were suppressed by E. schlechteranum [39]. The anti-HIV drug was discovered to be digitoxigenin-3-O-glucoside, a cardiac glycoside. Regardless of the fact cardiac glycosides are recognized for their toxicity, which may be connected to their anti-HIV effect, this chemical showed just a slight anticancer activity (20% suppression on Vero cells at 25 μg/mL) [33][36]. At 100 ng/mL, approximately 90% of the recombinant virus was inhibited.4.7. Anti-plasmodial Activity
Using the parasite lactate dehydrogenase test, Nethengwe et al. examined the anti-plasmodial effects of E. transvaalense bark of dichloromethane, methanolic, and aqueous extracts against Plasmodium falciparum the chloroquine-sensitive strain of (D10). Excluding dichloromethane, which had an IC50 of 5.1 μg/mL, the other extracts were inactive [40]. These findings supported the idea that E. transvaalense might be a source of antimalarials and, to a certain extent, back up the species’ historical usage as an herbal treatment for fever and malaria [40].4.8. Larvicidal Activities
Using the mosquito larvicidal assay of Culex quinquefascitus larvae, Nethengwe et al. examined the larvicidal properties of E. transvaalense bark of dichloromethane, methanolic, and aqueous extracts. The percentage mortality of Culex quinquefascitus fourth instar larvae revealed that aqueous extracts (35%) had the least larvicidal action, followed by methanol (47%) and dichloromethane (60%). Methanol and dichloromethane extracts had IC50 values of 9.8 µg/mL and 18.2 µg/mL, respectively [40]. These data confirmed the utilization of E. transvaalense as an anti-malarial herbal medication [40].4.9. Anti-pyretic Activities
Nethengwe et al. investigated the anti-pyretic effects of E. transvaalense bark of methanolic and dichloromethane extracts in male and female Sprague-Dawley rats, using paracetamol as a control medication. The extracts reduced pyrexia in the provoked rats. Their effects were concentration and time course-dependent, with the extracts exhibiting action as soon as thirty minutes, even at the least dose of 100 mg/kg. The activity of the methanol extract was equivalent to that of paracetamol, the reference medication [40]. These data reinforce the use of E. transvaalense as a fever-fighting herbal medication.4.10. Hypoglycaemic Activity
The inhibitory effects of E. transvaalense stem bark acetone extract on carbohydrate-hydrolysing enzymes α-glucosidase and α-amylase on hypoglycaemic activity were researched by Deutschländer et al. By assessing glucose absorption, the acetone extracts were tested against Chang liver, C2C12 myocyte, and 3T3-L1 preadipocyte cells. At 50 μg/mL concentration, the extracts demonstrated a 138.6% potential to reduce blood glucose levels in 3T3-L1 preadipocytes in an in vitro experiment. The extracts’ 50% IC50 for α-glucosidase and α-amylase were reported to be 50.6 µg/mL and 1.1 μg/mL, correspondingly [41]. These results demonstrate the use of E. transvaalense as an antidiabetic herbal medication [41].4.11. Anti-arthritic Activity
Using an anti-protein denaturation experiment, Elisha et al. examined the anti-arthritic effects of E. croceum acetone leaves extract. In an in vitro anti-arthritic test, the extract displayed an amount of the drug response, with an IC50 value of 80.0 μg/mL, greater than the positive control diclofenac sodium’s IC50 value of 32.4 µg/mL [30]. The extracts’ promising properties back up the species’ longstanding use for inflammatory diseases [30].4.12. Anti-diabetic Activity
In an alloxanized rat model, Lanjhiyana et al. investigated the anti-diabetic effect of stem bark methanolic extract of E. glaucum [17]. The goal of the investigation was to quantify the total phenolic content of ED methanolic extract (MED) and assess its antidiabetic potential in normal and alloxan-induced diabetic rats. The trial employed inbred adult male Charles-Foster (CF) albino rats for antidiabetic activity in OGTT and nondiabetic rats, as well as antidiabetic activity in alloxan-induced rats. MED responded positively for carbohydrates, flavonoids, alkaloids, tannins saponins, triterpenes, and sterols, according to phytochemical analysis. The MED also revealed a total phenolic content of 285.2 mg/g. In diabetic control experimental rats, the increasing level of glycosylated hemoglobin (HbA1c) is exactly proportionate to the reduced level of total hemoglobin. For assessing the degree of protein glycation during diabetes mellitus, glycosylated hemoglobin (HbA1c) is utilized as the most accurate marker and standard diagnostic technique. Protein glycation is a non-enzymatic process that occurs when excess glucose in the blood reacts with free amino groups on hemoglobin’s globin component. The HbA1c level is used to determine long-term glycemic status and to connect with different problems associated with diabetes. In experimental rats, oral treatment with MED dramatically reduced HbA1c levels, probably due to normoglycemic control mechanisms, which also reflected lower protein glycation condensation reactions, and the results were consistent with prior findings [17]. The continuing post-treatment with MED for 21 days demonstrated potential hypoglycemic action in OGTT and normoglycemic rats, as well as antidiabetic activity in alloxan-induced rat models, according to the findings. This suggests that plants may have an insulin-like function, which might assist in lowering the risk of lipid-related problems. Significant lipid management may help to prevent the coexistence of hypercholesterolemia and hypertriglyceridemia, as well as lower cardiovascular risk factors [17].References
- Opiyo, S.A.; Manguro, L.O.A.; Owuor, P.O.; Ateka, E.M. Triterpenes from Elaeodendron schweinfurthianum and their antimicrobial activities against crop pathogens. Am. J. Chem. 2017, 7, 97–104.
- Archer, R.H.; Van Wyk, A.E. A taxonomic revision of Elaeodendron Jacq. (Cassinoideae: Celastraceae) in Africa. S. Afr. J. Bot. 1998, 64, 93–109.
- Cao, S.; Brodie, P.J.; Miller, J.S.; Ratovoson, F.; Callmander, M.W.; Randrianasolo, S.; Rakotobe, E.; Rasamison, V.E.; Suh, E.M.; TenDyke, K.; et al. Antiproliferative Cardenolides of an Elaeodendron Sp. from the Madagascar rain forest. J. Nat. Prod. 2007, 70, 1064–1067.
- Yelani, T.; Hussein, A.A.; Meyer, J.J.M. Isolation and identification of poisonous triterpenoids from Elaeodendron croceum. Nat. Prod. Res. 2010, 24, 1418–1425.
- Kubo, I.; Fukuhara, K. Elabunin, a new cytotoxic triterpene from an east African medicinal plant, Elaeodendron buchananii. J. Nat. Prod. 1990, 53, 968–971.
- Osorio, A.A.; López, M.R.; Jiménez, I.A.; Moujir, L.M.; Rodríguez, M.L.; Bazzocchi, I.L. Elaeodendron orientale as a source of cytotoxic Cardenolides. Phytochemistry 2014, 105, 60–67.
- Tshikalange, T.E.; Hussein, A.A.; Meyer, J.J.M. Antimicrobial activity, toxicity and the isolation of a bioactive compound from plants used to treat sexually transmitted diseases. J. Ethnopharmacol. 2005, 96, 515–519.
- Omwenga, E.O.; Hensel, A.; Pereira, S.; Shitandi, A.A.; Goycoolea, F.M. Antiquorum sensing, antibiofilm formation and cytotoxicity activity of commonly used medicinal plants by inhabitants of Borabu Sub-County, Nyamira County, Kenya. PLoS ONE 2017, 12, e0185722.
- Omara, T.; Kiprop, A.K.; Ramkat, R.C.; Cherutoi, J.; Kagoya, S.; Moraa Nyangena, D.; Azeze Tebo, T.; Nteziyaremye, P.; Nyambura Karanja, L.; Jepchirchir, A.; et al. Medicinal plants used in traditional management of cancer in Uganda: A review of ethnobotanical surveys, phytochemistry, and anticancer studies. Evid.-Based Complement Altern. Med. 2020.
- Lemmens, R.H.M.J.; Louppe, D.; Oteng-Amoako, A.A. Timbers 2. Plant Resources of Tropical Africa-PROTA; PROTA Foundation: Wageningen, The Netherlands, 2012; Volume 2, ISBN 9789290814955.
- Odak, J.A.; Manguro, L.O.A.; Wong, K.C. New compounds with antimicrobial activities from Elaeodendron buchananii stem bark. J. Asian Nat. Prod. Res. 2018, 20, 510–524.
- Lemmens, R.H.M.J. Elaeodendron buchananii (PROTA)-PlantUse English. Available online: https://uses.plantnet-project.org/e/index.php?title=Elaeodendron_buchananii_(PROTA)&mobileaction=toggle_view_desktop (accessed on 21 September 2022).
- Nguta, J.M.; Mbaria, J.M.; Gakuya, D.W.; Gathumbi, P.K.; Kiama, S.G. Antimalarial herbal remedies of Msambweni, Kenya. J. Ethnopharmacol. 2010, 128, 424–432.
- Tsujino, Y.; Ogoche, J.I.J.; Tazaki, H.; Fujimori, T.; Mori, K. Buchaninoside, a steroidal glycoside from Elaeodendron buchananii. Phytochemistry 1995, 40, 753–756.
- Maroyi, A. Medicinal uses, phytochemistry, pharmacology and toxicological properties of Elaeodendron croceum. Trop. J. Pharm. Res. 2019, 18, 669–676.
- Mamba, P.; Adebayo, S.A.; Tshikalange, T.E. Anti-microbial, anti-inflammatory and HIV-1 reverse transcriptase activity of selected South African plants used to treat sexually transmitted diseases. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1870–1876.
- Lanjhiyana, S.; Garabadu, D.; Ahirwar, D.; Bigoniya, P.; Chand, A. Antidiabetic activity of methanolic extract of stem bark of Elaeodendron glaucum Pers. in alloxanized rat model. Adv. Appl. Sci. Res. 2011, 2, 47–62.
- Tsanuo, M.K.; Hassanali, A.; Jondiko, I.J.O.; Torto, B. Mutangin, a dihydroagarofuranoid sesquiterpene insect antifeedant from Elaeodendron buchananii. Phytochemistry 1993, 34, 665–667.
- Jayaweera, D.M.A. Medicinal Plants (Indigenous and Exotic) Used in Ceylon: Part II; The National Science Foundation: Colombo, Sri Lanka, 2006.
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1934578X19850354.
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive compounds and health benefits of edible rumex species-A review. Cell. Mol. Biol. 2018, 64, 27–34.
- Omosa, L.K.; Amugune, B.; Mutai, P.; Karumu, E.; Mukungu, N.; Induli, M.; Kama-Kama, F.; Kuete, V. Rapid screening using GIBEX screens-to-nature system of ethnomedicinal plants from Ngong Forest, Kenya for potency against infectious diseases and antioxidant activities: A qualitative study. Pharmacogn. Commun. 2019, 9, 59–74.
- Maregesi, S.M.; Hermans, N.; Dhooghe, L.; Cimanga, K.; Ferreira, D.; Pannecouque, C.; Berghe, D.A.V.; Cos, P.; Maes, L.; Vlietinck, A.J.; et al. Phytochemical and biological investigations of Elaeodendron schlechteranum. J. Ethnopharmacol. 2010, 129, 319–326.
- Maroyi, A.; Semenya, S.S. Medicinal uses, phytochemistry and pharmacological properties of Elaeodendron transvaalense. Nutrients 2019, 11, 545.
- Núñez, M.J.; Ardiles, A.E.; Martínez, M.L.; Torres-Romero, D.; Jiménez, I.A.; Bazzocchi, I.L. Triterpenoids from Cassine xylocarpa and Celastrus vulcanicola (Celastraceae). Phytochem. Lett. 2013, 6, 148–151.
- Osorio, A.A.; Muñóz, A.; Torres-Romero, D.; Bedoya, L.M.; Perestelo, N.R.; Jiménez, I.A.; Alcamí, J.; Bazzocchi, I.L. Olean-18-ene triterpenoids from Celastraceae species inhibit HIV replication targeting NF-KB and Sp1 dependent transcription. Eur. J. Med. Chem. 2012, 52, 295–303.
- Araujo-León, J.A.; Cantillo-Ciau, Z.; Ruiz-Ciau, D.V.; Coral-Martínez, T.I. HPLC profile and simultaneous quantitative analysis of tingenone and pristimerin in four Celastraceae species using HPLC-UV-DAD-MS. Rev. Bras. Farmacogn. 2019, 29, 171–176.
- Odeyemi, S.W.; Afolayan, A.J. Biological activities and phytochemical screening of Elaeodendron croceum (Thunb.) DC. leaves and stem barks extracts. Int. J. Phytomedicine 2017, 9, 566.
- Olaokun, O.O.; Mkolo, N.M.; King, P.H. Inhibition of 5-lipoxygenase and phytochemical content of five South African medicinal plants. S. Afr. J. Bot. 2018, 115, 326–327.
- Elisha, I.L.; Dzoyem, J.P.; McGaw, L.J.; Botha, F.S.; Eloff, J.N. The anti-arthritic, anti-inflammatory, antioxidant activity and relationships with total phenolics and total flavonoids of nine South African plants used traditionally to treat arthritis. BMC Complement Altern. Med. 2016, 16, 307.
- Khumalo, G.P.; Sadgrove, N.J.; Van Vuuren, S.F.; Van Wyk, B.E. Antimicrobial lupenol triterpenes and a polyphenol from Elaeodendron transvaalense, a popular Southern African medicinal bark. S. Afr. J. Bot. 2019, 122, 518–521.
- McGaw, L.J.; Jäger, A.K.; Van Staden, J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J. Ethnopharmacol. 2000, 72, 247–263.
- Prinsloo, G.; Meyer, J.J.M.; Hussein, A.A.; Munoz, E.; Sanchez, R. A Cardiac glucoside with in vitro anti-HIV activity isolated from Elaeodendron croceum. Nat. Prod. Res. 2010, 24, 1743–1746.
- Elisha, I.L.; Dzoyem, J.P.; Botha, F.S.; Eloff, J.N. The efficacy and safety of nine South African medicinal plants in controlling Bacillus anthracis sterne vaccine strain. BMC Complement Altern. Med. 2016, 16, 5.
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement. Altern. Med. 2017, 17, 133.
- Prinsloo, G.; Meyer, J.J.M.; Hussein, A.A. Anti-HIV activity of a cardiac glycoside isolated from Elaeodendron croceum. S. Afr. J. Bot. 2007, 73, 308.
- Pauwels, R.; Balzarini, J.; Baba, M.; Snoeck, R.; Schols, D.; Herdewijn, P.; Desmyter, J.; De Clercq, E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 1988, 20, 309–321.
- Pannecouque, C.; Daelemans, D.; De Clercq, E. Tetrazolium-based colorimetric assay for the detection of HIV replication inhibitors: Revisited 20 years later. Nat. Protoc. 2008, 3, 427–434.
- Bessong, P.O.; Obi, C.L.; Andréola, M.L.; Rojas, L.B.; Pouységu, L.; Igumbor, E.; Meyer, J.J.M.; Quideau, S.; Litvak, S. Evaluation of selected South African medicinal plants for inhibitory properties against human immunodeficiency virus type 1 reverse transcriptase and integrase. J. Ethnopharmacol. 2005, 99, 83–91.
- Nethengwe, M.F.; Opoku, A.R.; Dludla, P.V.; Madida, K.T.; Shonhai, A.; Smith, P.; Singh, M. Larvicidal, antipyretic and antiplasmodial activity of some Zulu medicinal plants. J. Med. Plants Res. 2012, 6, 1255–1262.
- Deutschländer, M.S.; van de Venter, M.; Roux, S.; Louw, J.; Lall, N. Hypoglycaemic activity of four plant extracts traditionally used in South Africa for diabetes. J. Ethnopharmacol. 2009, 124, 619–624.
