Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ying Liu and Version 2 by Catherine Yang.
Rubber composites are extensively used in industrial applications for their exceptional elasticity. The fatigue temperature rise occurs during operation, resulting in a serious decline in performance. Reducing heat generation of the composites during cyclic loading will help to avoid substantial overheating that most likely results in the degradation of materials. Influencing factors of heat generation are highlighted, including the Payne effect, Mullins effect, interface interaction, crosslink density, bond rubber content, and fillers.
- heat generation
- heat build-up
- rubber composites
1. Payne Effect
The storage modulus of unfilled rubber depends on the temperature and frequency of dynamic loads, which have nothing to do with the deformation amplitude of the rubber. Instead, the storage modulus for filled rubber depends on dynamic deformation, and the storage modulus value reduces noticeably as strain amplitude increases. The presence of a filler network in rubber composites above the percolation threshold can be blamed for this behavior, known as the Payne effect [1][25].
The amplitude dependency of the dynamic properties of composites is known as the Payne effect, which relates to a nonlinear property in the storage modulus of materials that decreases with the amplitude of dynamic strain. ∆E′ = E′0.1% − E′10% represents the Payne effect [2][11].
The modulus of the filled nature rubber composites steeply drops with increasing strain and expresses a typical nonlinear behavior. The Payne effect is mainly bound up with the filler network produced in the rubber matrix. Taking silica filler as an example, the rubber trapped between the fillers would lose activity, and the filler–filler network is increased. Therefore, the effective volume of the filler would sharply increase upon filler networking. Nevertheless, when the natural astaxanthin modifies the filler, the distribution and dispersion of the silica in the rubber matrix are obviously improved because of the weakened polarity of the silica surface, and the Payne effect is lowered [3][26]. The Payne effect does not appear to affect the structural evolution of the filler agglomeration, as evidenced by the fact that the destruction and recovery of carbon agglomerates do not coincide with the emergence of the Payne effect [4][27].
2. Mullins Effect
The Mullins effect describes the stress reduction among the initial loading and successive reloading of the loaded rubber under quasi-static cyclic loading [5][18]. The Mullins effect [6][28] is caused by irreversible structure changes, such as the breaking of chain segments and the separation of fillers.
The addition of fillers evidently increases the viscoelastic character of the rubber matrix. The strain cannot catch up with the change in stress under the cyclic loading situation, which results in a clear hysteresis ring (Figure 12). The Mullins effect and filler network structure go hand in hand during the cyclic loading [7][29].
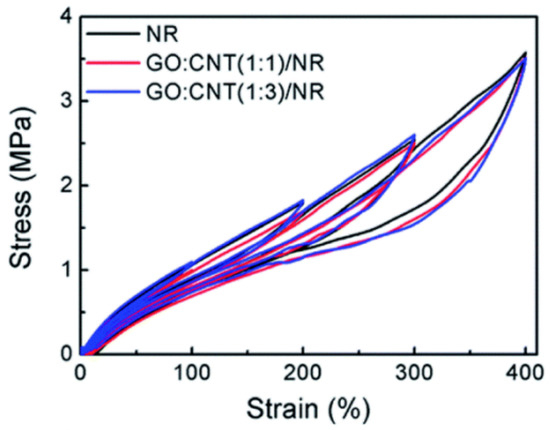
The loss modulus is decided by the loading cycle number, which can be ascribed to cyclic stress relaxation. This is owed to the chain slipping and migration of entanglement [8][30]. The decreasing of loss modulus is expressed as the dynamic softening effect by Li Fanzhu and his coworkers [9][10], which is similar to the Mullins effect, then the relationship is established between the loading cycle number and loss modulus to characterize the energy dissipation rate.
3. Interface Interaction
The interfacial properties between the filler and rubber matrix play an essential role in the properties of rubber composites [10][31]. The interfacial interaction between fillers and polymer chains is a key factor in enhancing rubber performance [11][32]. The polymer–filler interaction can be measured by the energy loss fraction of the polymers, tan δ. For the viscoelastic behavior, the relationship of the energy loss fraction, W, of the rubber composite is expressed as follows [12][13][33,34],
The energy loss fraction W at the tan δ is given by the following equation,
where C is the volume fraction of the constrained region, (1 − C) is the fraction of the amorphous region, and W0 and C0 denote the energy fraction loss and volume fraction (C0 is taken to be 0) of the constrained region for rubber blends, respectively. It can be rearranged as below,
The polymer–filler interaction increases with the value of C.
One of the most important problems is the dispersibility of the filler particles in the rubber matrix. The surface energy of the filler has determined the wetting of the filler by the polymer matrix [1][25]. Phonon scattering at the filler–polymer interface can be minimized by the creation of a strong covalent bond between the modified fillers and the polymer [14][35]. The generated heat inside quickly transmits to the surrounding environment owing to the high thermal conductivity; hence, the temperature of the rubber composites significantly decreases.
Studies on the nonlinear viscoelasticity and thermal conductivity of the B4C/NR composites demonstrate that the well-maintained and highly thermally conductive channels are the primary cause of the increased thermal conductivity [15][36]. There are two main reasons for the addition of B4C significantly reducing the heat generation: (1) heat has been conducted outside more effectively due to the increased thermal conductivity, and (2) the weak interfacial activity and low surface area decrease the interfacial friction effectively [15][36].
To improve the bonding strength between polymer matrix and filler, hydroxyl telechelic natural rubber (HTNR) with hydroxyl-terminated groups has been introduced into silica-reinforced natural rubber by Katueangngan et al. [16][37]. The increase in temperature rising with silica loading is a result of the silica–silica network and the poor dispersion of filler particles in rubber composites. Nevertheless, the addition of HTNR significantly decreases temperature rise. Adding HTNR can improve the dispersion of silica and reduce the silica–silica network. Thus, the re-agglomeration of filler is decreased, resulting in less heat generation. Furthermore, the reduction in heat generation is also owing to strengthened rubber–filler interactions through the hydroxyl groups of HTNR interacting with the silanol groups on silica surfaces.
Nanospring-filled elastomer composites have been constructed for two types (Figure 23): nanosprings and polymer chains have been directly blended to obtain system I; system II, on the other hand, is a tri-block chain–nanospring–chain structure that self-assembles. The findings suggested that the permanent deformation of composites could be reduced by the chemical connections at the interface between nanosprings and polymer chains. The good dispersion and interfacial interaction between the nanosprings and the polymer chains can significantly decrease the hysteresis loss of composites [17][38].
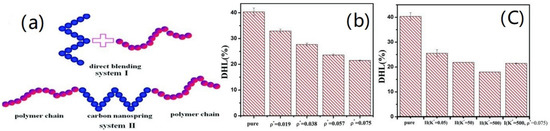
The relationship of crosslink density and entanglement points with the thermogenesis properties of NR was proposed by Yue-Hua Zhan [18][39]. They found that the crosslink and entanglement points can hold the rubber chain to restrict the movement of the polymer chain and reduce heat generation.
Lai et al. used the latex compounding ingredients without a strong reducing agent to enhance the thermal conductivity of NR/graphene composite properties by the NR/rGO interaction [19][40].
4. Crosslink Density
When uncrosslinked rubber composites are stressed, molecular chains can easily slide past each other and disentangle. A few crosslinks can increase the molecular mass, have a broader molecular mass distribution, and create branched molecules. It is much harder for these branched polymers to disentangle, and hence, tensile strength of the crosslinked elastomer increases. When a rubber composite is deformed by an outside force, parts of the energy lied in the polymer chains and can be used as a driving force for fracture. The leaving energy is dissipated through polymer movement into heat and cannot break chains. High crosslinking may restrict chain motions, and the network cannot release much energy.
The heat build-up property of elastomers depends strongly on crosslink density. Hysteretic effects decrease monotonically with increasing crosslink density. There is an optimal crosslink density for practical use. High enough crosslink densities should prevent viscous flow failure, whereas low enough densities should prevent brittle failure [20][15].
Fang and coworkers [21][14] studied the crosslink density of rubber compounds filled with different amounts of Si-69. The networks among coupling agent, silica, and polymers become more and more intensive, which increases the crosslink density of rubber compounds. When the crosslink density is increased, the loss modulus and dynamic lag loss of the sample show a declining trend.
Heat build-up and crosslinking have an important relationship [22][41]. A higher degree of network stability usually causes lower heat generation. A low degree of heat build-up can be expected by the comparison of heat generation tests before and after aging.
5. Bound Rubber
The interaction between polymer and filler determined by the types and numbers of adsorption sites determines the interfacial structure between the two [23][42]. At room temperature, bound rubber is a term used to describe polymer chains that a suitable solvent cannot remove from suspensions. Bound rubber also serves as an indirect indicator of how the polymer and filler interact.
On the surface of fillers, the ‘‘bound rubber layer’’ is composed of neighboring constrained chains and adsorbed chains. The polymer mass has a great influence on the thickness of the layer [2][11]. Unperturbed chains and restricted chains are combined to create the “constrained rubber layer” on the external layer. The inclusion of filler particles does not fundamentally impair the mobility of the unaffected chains that surround the confined rubber layer, also known as bulk rubber. Divide the layers with clear limits to simplify (Figure 34). For bound rubber limiting the movement of polymer chains, the energy loss caused by the glass transition of polymer is reduced [24].
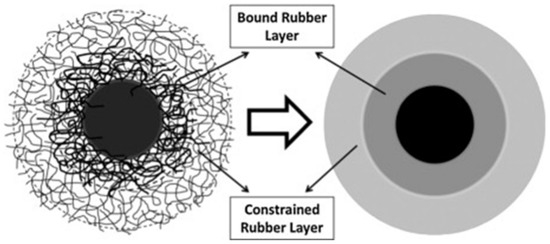
Figure 34. Schematic representation of interfacial structure between filler and polymer (bold lines mean adsorbed chains) [23].
Schematic representation of interfacial structure between filler and polymer (bold lines mean adsorbed chains) [42].
6. Fillers
Since most the rubber materials exhibit poor mechanical properties, the addition of high loading of fillers is mandatory to achieve satisfactory mechanical properties. However, the maximum amount of filler always brings about friction and temperature rise [25][43]. Filler size, loading, coupling agent, and synergistic effects all have an impact on how much heat is generated when fillers are added to rubbers.
6.1. Effects of Filler Size and Loading
According to the report, the deformation and reorganization of the aggregated filler particles under cyclic deformation are responsible for the temperature rise; in other words, the better dispersion of fillers or little filler–filler network could bring about less heat generation.
Carbon black has been extensively applied as a reinforcing filler for polymer composites [26][27][28][29][44,45,46,47]. Wongwitthayakool et al. [30][48] used various carbon black addition and performance (such as specific surface area and structure) to prepare hydrogenated acrylonitrile butadiene rubber (HNBR) composites. The amplitude of the temperature rise is more pronounced in specimens with high specific surface area and carbon black structure, and the temperature rise is greatly increased with carbon black loading and surface area rising. Graphene has a large specific surface area and corrugated structure; therefore, it can obstruct the rubber macromolecules from slipping along the surface of graphene; thus, the intrinsic friction and energy loss are reduced, and the internal heat rise is decreased [31][49].
Nature rubber with low surface and high carbon black structures exhibit low temperature rise [32][50]. The influence of carbon black loading on the performance of NR composites is studied by Zhang et al. [33][51], especially the temperature rise. The content of bound rubber increases with an increase of carbon black loading, which enhances the inner rub between the filler and polymer. Additionally, the free volume decreases in the process of the test, then the temperature rise progressively increases with filler loadings. In general, when fillers are used to reinforce rubber, the higher the filler content, the higher the heat accumulation of rubber composites [34][23]. Uraiwan Sookyung prepared NR/organoclay nanocomposites with various organoclay loadings. The results make known that the temperature rise increased with organoclay loading [35][52]. In theory, monodispersed filler particles that do not participate in agglomeration can adsorb more polymer chains. Increasing the number of filler particles can cut down the distance between particles, which would help the formation of bridging chains [23][42]. Hysteresis loss increases with an increase in dangling chain ends ratio by comparing the hysteresis loss for different rubbers [36][8].
6.2. Effects of Coupling Agent
The modified filler particle surfaces led to good distribution and compatibility among the matrix and the filler, which had a homogeneously dispersed system [14][37][35,53]. At the same filler loading, the better dispersion of the filler in the rubber matrix, the more filler–rubber interaction points will be formed [38][54].
Bo Yang et al. [39][55] developed silica/NR composites by co-modifying silica with dodecanol and the silane coupling agent KH-592 (Figure 45). The silane coupling agent KH-592 can create a bridge structure between the filler and matrix when a mercapto group is present, which enhances the interaction between the silica and NR matrix. As a result, there is less friction between the filler–filler and the filler–polymer interaction points, and the rubber composite material with low temperature rise is prepared.
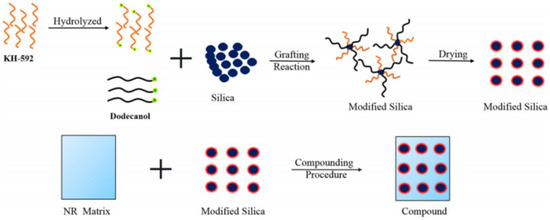
Figure 45. Flowchart for the production of silica/NR for co-modified silica particles with dodecanol and KH-592 [39].
Flowchart for the production of silica/NR for co-modified silica particles with dodecanol and KH-592 [55].
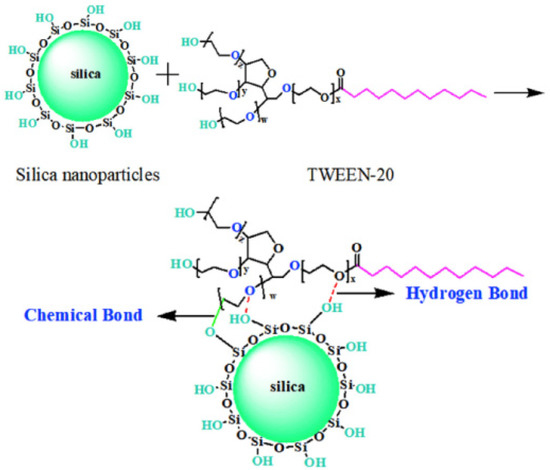
Xiao et al. [40][56] first use a new silica modifier TWEEN-20 (Figure 56), which has four long arms made up of three polyether chains with a terminal hydroxyl group and a fatty chain. A hydrogen bond is formed by the oxygen on the polyether and the silanol groups on the silica surface, and a chemical reaction may occur between the terminal hydroxyl and the silanol groups without any volatile organic compounds (VOCs). Besides that, to get good compatibility with the rubber matrix, the long fatty chain undercut silica polarity, so that silica modified by TWEEN-20 can homogeneously disperse in the polymer matrix. Therefore, the addition of TWEEN-20 can significantly reduce the HBU of the composites.
A graphene oxide (GO)-supported vulcanization accelerator (NS-rGO) is prepared by Cheng et al. [34][23] through a reaction of the vulcanization accelerator GO and N-tert-butyl-2-benzothiazole sulfonamide (NS). The temperature rise of the sample only is 2.6 °C when the amount of NS-rGO is 0.42 vol%, and it decreased by 0.9 °C compared with the neat nature rubber. It is due to that the heat generation in rubber is mostly produced by the friction between networks, for example, polymer–polymer, filler–filler, and filler–polymer networks. Under dynamic loading, NS is the bridge to connect GO and nature rubber through the strong chemical bonds to prevent the NR from sliding along the surface of GO. The dynamic friction between the fillers is reduced, and the friction between fillers and matrixes is also reduced, as is the heat generation. It shows that the validly chemical bonds among the fillers and polymer chains can actually decrease the heat generation of rubber compounds.
6.3. Synergistic Effect
Research has found that heat transfer can be significantly improved by introducing a combination of different kinds of filling materials, such as micron and nanometer fillers. Compared with a single size and shape, the synergistic effect of multiple structures makes it more concise to form effective thermal conductive pathways in a polymer matrix [41][42][57,58]. Hybridization of nanofillers is a new method to promote the uniform dispersion of nanofillers in polymer matrices. To get excellent properties of synergistic effects, at least two different fillers will be plugged into the polymer matrix [43][44][59,60]. Jafarpour et al. found that the hybridization of carbon nanotube (CNT) and nanodiamond (ND) enhanced the stability of individual nanoparticles in styrene-butadiene rubber (SBR) and the thermal conductivity of composites in a synergistic manner [44][60].
In SBR/CB composites, the disaggregation and re-agglomerate of CB cause friction among CB particles and bring about vast amounts of energy dissipation in the form of heat. The results have shown that the addition of MoS2 has a good function in improving the dispersity of CB. MoS2 is a good lubricant to reduce the friction coefficient between MoS2 sheets and CB. The addition of 3 phr MoS2 leads to a 10 °C decrease in the temperature rise of the composites [45][61].
Mohanty et al. [46][62] used clay partly to replace CB can increase the temperature rise of SBR/CB composites. With the replacement of CB by nanoclay, the possibility of CB–nanoclay interface formation will increase leading to the increase of the inter-filler friction, ultimately resulting in the loss of energy in form of heat.
Due to GO’s surfactant properties and the potent p–p interaction with CNTs and GO, Li et al. [47][63] mixed up the CNTs with GO aqueous solution to obtain stable hybrid suspensions. It turns out that the GO/CNTs hybrid fillers can produce a unique three-dimensional filler network in a natural rubber matrix. This unique filler network can be used as a sacrificial bond to utilize energy before the material breakdown, which significantly lowers temperature rise in GO/CNTs/NR composites.
Graphene can inhibit the “flow” of carbon black and reduces the strain phase lag and loss factor of the composites. Enhancing the dispersion of filler and interface interaction of the composite can bring about the interfacial stress transfer more quickly. Hence, the modified GO and CB-filled NR can show brilliant dynamic mechanical properties [2][11].
Wei et al. [7][29] used GO and CNT hybrid fillers to replace partial CB (Figure 67). Parts of CNT and CB are absorbed on the surface of the GO sheet so it can produce a hybrid network beneficially. The synergistic intercalation between GO, CNTs, and CB could arrest their re-agglomeration and form a developed network. Therefore, the hybrid fillers enhance the dispersity of CB in the NR matrix. The temperature rise of GO/CNTs/CB/NR composites is less than CB/NR, which indicates that the excellent dispersing performance of CB, and the effective filler network can effectively improve the thermal conductivity of NR composites, resulting in the less temperature rise compared to CB/NR. Therefore, with the hybrid fillers affiliating, the temperature rise of CB/NR composites is markedly decreased.
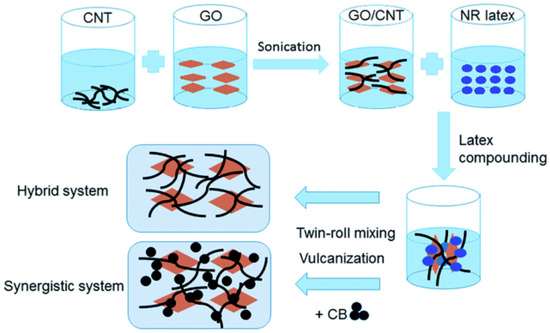
Figure 67. Latex mixing process utilized to create the GO/CNT/NR composites is depicted schematically [7].
Most of the papers test the bottom of the sample to obtain the temperature rise as shown in Figure 78, and only a few researchers test the center point of the sample. The lowest temperature rise of 7 °C can be measured when the amount of filler is 3.5 phr, but the tensile strength is only 13.3 MPa [15][36].
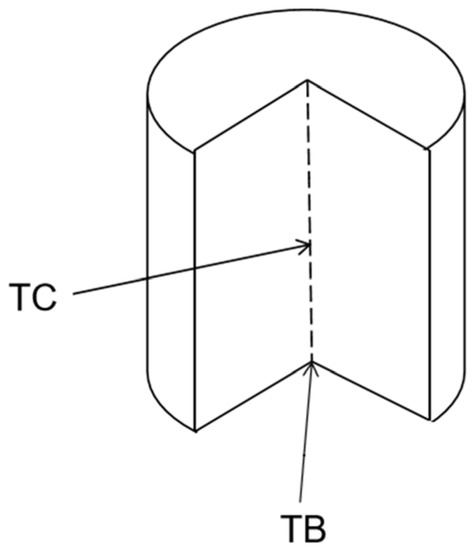
Figure 78.
Description of test position.
To sum up, the kind of fillers, particle size, filler loading, synergistic effect, and coupling agent can have a great influence on HBU. To obtain composites with the lower temperature rise, lower Payne effect, lower Mullins effect, stronger interface interaction between fillers and polymer chains, much more bound rubber and higher crosslink density are required.
Heat built-up, the heat generated by internal friction in the rubber, is only related to rubber components and interactions between components. It is also related to the loading peculiarity, such as frequency, amplitude, time, etc.
Temperature rise is a comprehensive reflection of the heat transfer inside the specimen between the specimen and between the specimen with the surrounding air. Therefore, the temperature rise is related to the heat built-up and thermal conductivity of composites. Raising the thermal conductivity of rubber composites is profitable for the diffusion of heat and the decrease of the temperature rise [37][53]. As a result, whether the composites can dissipate heat in time is the major factor for the normal service of rubber products. The primary and internal factors that determine the thermal conductivity coefficient values of polymers and polymer composites are typically chain structures of polymer matrix materials, interfacial thermal resistances, and intrinsic thermal conductivity coefficient values of thermally conductive fillers [48][68]. The improvements in thermal conductivity of polymer materials are very important for their application as thermal interface materials (TIMs) [49][50][69,70] and electromagnetic screening materials [51][71].
