Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Luana Coelho and Version 2 by Catherine Yang.
Different strategies involving biopolymers, blends, nanotools, and immobilizing systems have been studied against infected wounds. Lipids of animal, mineral, and mainly vegetable origin have been used in the development of topical biocompatible formulations, since their healing, antimicrobial, and anti-inflammatory properties are interesting for wound healing. Vegetable oils, polymeric films, lipid nanoparticles, and lipid-based drug delivery systems have been reported as promising approaches in managing skin wounds. Carbohydrate-based formulations as blends, hydrogels, and nanocomposites, have also been reported as promising healing, antimicrobial, and modulatory agents for wound management.
- carbohydrates
- healing process
- infected wounds
- lipids
- proteins
- wound management
1. Proteins
Tissue repair, also named fibrogenesis, is a complex process orchestrated by different cell lines (inflammatory cells, epithelial cells, and myofibroblasts) and the extracellular matrix, in response to the wound healing process [1][45]. Various proteins are important actors mediating cell–matrix and cell–cell interactions. For example, integrins facilitate the communication between non-parenchymal cells of the extracellular matrix, such as inflammatory cells and fibroblasts, and parenchymal cells [2][46]. Recent research results show that defensins are known for their excellent antibacterial activity, thus playing an essential role in the complex pathophysiological changes of diabetic wounds [3][47]. Collagen, one of the most abundant proteins in the extracellular matrix, is an important source of elasticity and strength in the extracellular matrix and contributes to the structural and physiological integrity of tissues. This protein plays an important role in regulating the wound healing process by attracting fibroblasts and supporting the formation of new tissue in the wound bed [4][48].
Silk sericin (SS), one of the two major proteins forming the silkworm cocoon, in this case the Bombyx mori cocoon, was used to synthesize a hydrogel by repetitive freezing–thawing. SS, poly (vinyl alcohol) (PVA), and azithromycin (AZM) were crosslinked with genipin (GNP) and tested towards S. aureus, P. aeruginosa, E. coli, and Candida albicans. The hydrogel also showed sustained SS and AZM releases, cytocompatibility on keratinocytes and fibroblasts, as well as skin adhesion ability when freeze-dried. Regarding specifically the in vivo study using an infected mouse full-thickness burn model with a 10% total body surface area, the hydrogel presented a better burn wound healing effect than the commercial Tegaderm™ film dressing, thus minimizing systemic burn effects [5][49].
Antibacterial hydrogels, such as the above-mentioned AZM-hydrogel composed of SS, PVA, and GNP, are receiving increasing attention in the aspect of bacteria-infected-wound healing. However, biofilm infections and bacterial drug resistance leads to the hard healing of wounds, thus the elaboration of alternatives that can circumvent these issues is essential. For example, Ouyang et al. [6][50] cross-linked the green industrial microbicide tetrakis (hydroxymethyl) phosphonium sulfate (THPS) with model protein bovine serum albumin (BSA) to form an antibiotic-free protein hydrogel with excellent biocompatibility and superior antibacterial activity against drug-resistant bacteria and biofilms. When tested as a wound dressing in an in vivo study, the BSA-Hydrogel accelerated the reepithelization of MRSA-infected skin wounds without inducing significant side effects. The results were reported as a way to provide a facile, feasible, and general gelation strategy with promising applications in hospital and community MRSA disinfection and treatment.
Another silk hydrogel was developed with the purpose to avoid polymicrobial infections in infected diabetic wound ulcers. The dressing was produced with the silk hydrogel and a combined therapeutic effect of metal chelating dipeptide (L-carnosine) and curcumin. The results from cell viability revealed that the designed hydrogel matrix was compatible to human cells and significantly accelerated the diabetic healing potential. Additionally, the hydrogel presented bacterial inhibition against S. aureus and E. coli via in vivo mice wound sites, which indorsed the diabetic wound healing efficiency in streptozotocin-tempted diabetic mice [7][51].
Another example of nanocomposite hydrogel was fabricated with oxidized alginic acid, dopamine, and antimicrobial peptide ε-polylysine. The system was then cross-linked with acrylamide and tested against gram-positive and gram-negative bacteria; its hemostatic and adhesive properties were also tested. The designed hydrogel showed excellent bacterial inhibition and, when compared with a commercial alginate sponge, accelerated the healing of infected full-thickness wounds by reducing inflammation and promoting angiogenesis and collagen deposition. Therefore, the authors highlighted the nanocomposite as a multifunctional dressing for promoting the healing of infected wounds [8][52].
Gelatin is the hydrolysis product of the well-known collagen protein derived from connective tissues in animal skin, bone, and tendon. Due to their biocompatibility, convenience for chemical modifications, and degradability, gelatin-based hydrogels have a broad range of biomedical fields. However, their poor antibacterial ability hinders the application of gelatin hydrogels in treating infected wounds. Tao et al. [9][53] developed a series of multifunctional hydrogels based on gelatin methacrylate (GelMA) and dopamine methacrylate (DMA), both of them immersed into zinc nitrate solutions. The obtained wound dressings showed strong antibacterial activity against E. coli, good cytocompatibility, and enhanced adhesion, proliferation, and migration of NIH-3T3 cells. With the promising results, authors described Zn-incorporated hydrogels as bioactive materials potentially suggested as wound dressings for infected full-thickness wound healing and skin regeneration.
A gelatin hydrogel incorporated with bio-nanosilver (silver nanoparticles from a spent mushroom substrate) functionalized with lactoferrin (LTF) was prepared as a dual-antimicrobial action dressing. It was synthesized, characterized, and tested against S. aureus and P. aeruginosa, thus demonstrating an adequate release of nanoparticles and LTF, with promising antimicrobial effects against both bacterial strains. The authors concluded that the system was successfully synthesized as a new approach for fighting biofilms in infected wounds and thus may be applied to accelerate the healing of chronic wounds [10][54].
Not only collagen-containing systems but collagen itself is being prepared to boost its efficacy in wound healing. With presentations varying from particulate to powdered forms, this protein has been also tested as an adjunctive therapy for chronic wounds with indications, limitations, and principles of use. In general, the scientific literature reports a need for high quality studies and randomized control trials to support its use in clinical practice [11][55].
Different strategies involving biopolymers, nanotools, blends, and anti-inflammatory and antimicrobial immobilized drugs have been studied in nanofibers, nanoparticles, hydrogels, films, and sponges based on collagen. They have been recently reported as efficient alternatives in the healing of wounds, infected or not [4][48]. For example, a bilayer membrane composed of collagen, chitosan, Aloe vera, and gelatin was tested on a full-thickness skin wound model. The results of the evaluation revealed that the fabricated asymmetric membrane could facilitate wound healing by enhancing cellular activities and collagen deposition in addition to promoting proliferation within 10 days of treatment [12][56].
Fibrin, an important plasma protein involved in the coagulation process, was used as a hydrogel basis for the incorporation BNN6-loaded mesoporous polydopamine nanoparticles. After administration, the hydrogel created local hyperthermia and released large quantities of NO gas to treat the MRSA infection. Furthermore, the fibrin and small amount of NO originated from the hydrogel action promoted wound healing in vivo [13][57].
The use of peptides is an emerging alternative to conventional treatment strategies, including oral or parenteral antibiotics, in the treatment of wound infections. Antimicrobial peptides (AMPs), for example, are natural antimicrobials produced by plants, animals, fungi, protozoa, and bacteria that own potent antibacterial and wound healing effects. They are able to develop a sustained delivery approach for the control of the bacterial growth at the wound site; however, some limitations are reported (for example, instability related to oxidation, hydrolysis, and proteolysis) and then associated with a low residence time for topical applications to the wounds [14][15][58,59]. One way to circumvent these issues is the incorporation of AMPs in delivery platforms such as hydrogels [16][60]; their three-dimensional network, usually based on hydrophilic polymers, can enhance peptide stability and antimicrobial activity, as was reported by Thapa et al. [15][59] working with a hybrid hydrogel composed of Pluronic F127 (PF127), ethylenediaminetetraacetic acid (EDTA) loaded liposomes, glutathione (GSH), and the AMP from Lactococcus garvieae. Potent in vitro antibacterial and anti-biofilm effects were demonstrated against S. aureus, while the in vivo treatment of MRSA infected mouse wounds suggested potent antibacterial effects.
Nisin, the first known peptide with antimicrobial activity isolated from L. lactis in 1947, was purified, encapsulated in lipid nanoparticles, and then incorporated in a composite hydrogel wound dressing based on natural polysaccharides [gellan gum (GG) and a mixture of GG and alginate]. The most effective antimicrobial activity against S. pyogenes was observed for GG and nisin-loaded lipid nanoparticles [17][61]. AMPs derived from collagen were already obtained from marine sources, such as collagencin, which is derived from fish collagen [18][62].
An AMP-modified hyaluronic acid (HA-AMP) was prepared in a hydrogel system through Schiff’s base formation between the aldehyde group of oxidized-dextran and the amino group HA-AMP and platelet-rich plasma, and tested for the treatment of chronic infected wounds. The system exhibited significant inhibition zones against three pathogenic bacterial strains (E. coli, S. aureus, and P. aeruginosa) and the slow release of the modified AMP. The in vivo evaluation demonstrated that the hydrogel could significantly improve wound healing in a diabetic mouse infection by regulating inflammation and accelerating collagen deposition and angiogenesis [19][63].
Other differently sourced proteins were reported by the scientific literature dealing with wound healing therapy. For example, albumin, the most abundant plasma protein, essential in maintaining the osmotic pressure of plasma and transporting endogenous compounds (pigments, other proteins, and cholesterol), had its release behavior from the nanoparticles studied, and the suggestion for the on-demand antibacterial release upon application to infected wounds. Zein, a plant protein found in maize endosperm composed of four nonpolar amino acids (glutamine, leucine, proline, and alanine), was suggested as potent topical antibacterial formulations. However, no commercial products of zein for the topical antibacterial therapy of infected wounds were reported [20][64]. Therefore, considering the above-mentioned recent publications, proteins could be considered promising strategies for the development of formulations to treat wounds, including the infected ones.
2. Lipids
Lipids perform essential biological functions within the body, as a source of energy, as structural components of cell membrane, and as signaling molecules; their composition is associated with dietary fatty acid consumption. In the context of wound healing, lipids provide energy to the wound repair; they are responsible for providing substrates from lipids for wound cell proliferation and remodeling, highlighting that the consumption of adequate lipids is essential to the wound healing process [21][17]. In addition, lipids act as signaling agents during wound healing and tissue regeneration.
Many studies have reported the use of lipids, especially fatty acids, to manage wound healing [22][23][65,66]. Fatty acids are carboxylic acids with a long hydrocarbonate chain that compound the structural components of oils and fats, among other categories of lipids [24][67]. Vegetable oils are known to be a low-cost source of unsaturated fatty acids for the production of topically administered pharmaceutical products. Studies have reported the evaluation of omega-3, omega-6, and omega-9 in their free form and as blends on immune cell functions [25][68], and edible vegetable or animal oils on wound healing processes [26][27][69,70].
Vegetable oils have been reported to promote beneficial effects in cutaneous skin and especially in the wound healing process since their antimicrobial, antioxidative, and anti-inflammatory activities stimulate cell proliferation, reepithelization, and the reconstruction of the skin’s lipid barrier [27][28][70,71]. For example, virgin coconut oil was shown to be potent and fast healing when topically applied to treat excision wounds in young rats, evidenced by a decreased time of complete epithelization; significantly increased levels of total collagen, elastin, sialic acid, hexose, and DNA; a low level of lipid peroxidation; and an increase in fibroblast proliferation in treated wounds compared with control wounds [28][71]. Previous studies reported that coconut oil is rich in fatty acids of a medium chain length (6–12 C) [29][72], and these molecules may act in modulating cellular proliferation and cell signaling [30][73]. Thus, the wound healing properties of virgin coconut oil may be attributed, in part, to its fatty acids’ components. Other studies evaluated surgically induced wound closure evolution among rats treated with two oil blends: sunflower/canola oils 85/15 (BL1) and canola/linseed oils 70/30 (BL2); BL1 being administered during the inflammation phase (days 0–3), and BL2 in the tissue formation and remodeling phase (days 4–15). When compared with the control group treated with physiological saline, an increase in the areas of wounds treated with the blends in the inflammatory phase was observed, probably associated with the pro-inflammatory properties of the n-6 fatty acids present in BL1. Secondly, a steeper closure curve was observed, signaling a faster healing wound process resulting from the initial exacerbation of the inflammatory stage with the anti-inflammatory effects of the n-3 fatty acids from BL2 [31][74].
Beneficial effects for skin wound healing were also observed for Sapindus mukorossi seed oil, abundant in monounsaturated fatty acids. Wounds were created on the dorsum of rats and treated with a hydrogel based on carboxymethyl cellulose (CMC)/hyaluronic acid (HA)/sodium alginate (SA) for releasing the S. mukorossi seed oil [32][75]. Treated wounds exhibited an acceleration of the sequential skin wound healing process, including a higher decrease in size when compared with the untreated control. In addition, the oil showed significant anti-microbial activity against Propionibacterium acnes, S. aureus, and Candida albicans. Ferreira et al. [33][76] developed a biocompatible gel of chitosan associated with buriti oil (Mauritia flexuosa L.) to evaluate its potential to heal skin wounds. The lipid composition of buriti oil was also investigated, and it is basically composed of oleic and palmitic acids. Although buriti oil did not show antimicrobial activity, the composite chitosan-buriti oil evidenced potent action against S. aureus and Klebsiella pneumoniae at the 10 mg/mL. In addition, the composite gel also showed antioxidant activity and promoted faster and complete wound retraction, suggesting its potential for treating infected skin wounds. An oil extracted from Opuntia ficus indica L. inermis (OFI), composed by unsaturated fatty acids, triacylglycerols, phytosterols, and tocopherols [34][77], also demonstrated antimicrobial and wound healing potential [35][78]. An in vitro significant antimicrobial effect was observed against Enterobacter cloacae, C. parapsilosis, C. shake, and against the opportunistic Penicillium, Aspergillus, and Fusarium. OFI oil also showed potent wound healing, preventing cutaneous infections, and reducing the re-epithelialization phase in a rat model, suggesting a potential approach to treating infected wounds.
In the research to improve wound healing mechanisms, biological membranes have been evaluated to accelerate the healing, preventing infection and sepsis [36][79]. Frog (Rana tigerina) skins have shown a significant role in wound healing to induce proliferative activity of the epidermal and dermal cells. Frog skin lipid content is predominantly phospholipids and exhibits a dose dependent acceleration of wound healing, more specifically on acute inflammation and immunostimulatory response, whether topically applied or injected intraperitoneally [37][80].
Considering topical skin preparations that are commercially available, frequently they show the same limitations to impair wound healing, such as toxicity, drug instability, and a limited time of action [38][81]. Recently, topical forms containing nanostructured lipid carriers (Figure 14) represent a strategy to develop new products for wound care, mainly drug delivery systems, dressings, and films [21][17]. Considering the healing, antimicrobial, and anti-inflammatory properties of lipids, added to their solubility, stability, and plasticizing action, the lipids into formulations increase skin penetration and release the profile of the drugs, improving wound healing therapies compared with conventional treatments [23][66]. One research has reported the development of nanostructured lipid carriers based on cocoa butter as a solid lipid and olive oil as a liquid lipid to be loaded with eucalyptus oils and to be used to improve wound healing [39][82]. The surfactant chosen to stabilize the nanostructures was lecithin. Wound healing properties towards normal human dermal fibroblasts in vitro, and wound healing in vivo on a rat burn model, were characterized. These nanostructured lipid carriers showed good bioadhesion and cytocompatibility, adding to the enhancement of the in vitro and in vivo wound healing process and antimicrobial activity against S. aureus and S. pyogenes, two gram-positive bacteria commonly present in the skin. In this context, it is suggested that the high content of oleic acid, already known for its proliferation enhancement potential, associated with eucalyptus oil, promoted a synergic effect to wound repair and antimicrobial activity [40][83].
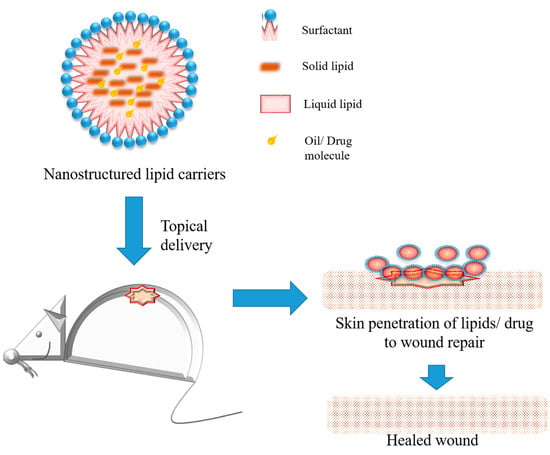
Figure 14.
Schematic representation of nanostructured lipid carriers, their composition, and application for wound healing on rat model.
Other studies evaluated the effect of lipid nanocarriers containing omega-3 fatty acid or liposomes containing omega-3 fatty acid and resveratrol in cotton textile substrates as dressings for wound healing. Considering the role on inflammatory response, both formulations inhibited nitric oxide (NO) production, demonstrating an anti-inflammatory effect that improved the wound healing process [22][65]. Films containing lipids have been developed for application in wound care, for example, chitosan films containing oleic acid or linoleic acid and glycerol [41][84], that were tested on wounds in burned patients, and it was evidenced that glycerol contributed to film adhesion, and the film promoted good epithelialization in a period of 12 to 15 days. Another chitosan film was prepared containing thyme oil for use as a healing dressing that showed potential antimicrobial and antioxidant activities, and was a promising approach for wound healing dressings [42][85].
In bacterial infected wounds there often occurs a disruption in the wound healing process, which is a challenge to treat, mainly on chronic pressure ulcers and diabetic wounds. Researchers have demonstrated that the antimicrobial lipids are broad-spectrum antibacterial agents, acting as immune innate responses and by specific mechanisms. Antimicrobial fatty acids act in the bacterial membrane, and the long-chain bases may inhibit cell wall synthesis [43][86]. Kim et al. [44][87] described the wound healing properties of a mix of 10 major lipid components of the Chamaecyparis obtusa extract. The 10-lipid mixture showed bactericidal effects against S. aureus and S. pyogenes, and protective effects against staphylococcal α-toxin-induced keratinocyte cell death. In addition, the 10-lipid mixture accelerated the healing of wounds superinfected with S. aureus in vivo.
Ghodrati, Farahpour, and Hamishehkar [45][88] conducted a study to evaluate the efficiency of peppermint essential oil (PEO) loaded into nanostructured lipid carriers (PEO-NLC) on in vitro antibacterial activity and in vivo infected wound healing in mice models. PEO and PEO-NLC demonstrated antibacterial activities against S. epidermidis, S. aureus, Lysteria monocytogenes, E. coli, and P. aeruginosa in vitro. In vivo analysis showed a greater wound contraction rate, fibroblast infiltration, collagen deposition, and re-epithelialization in PEO and PEO-NLC-treated animals when compared with the control group. Thakur et al. [46][89] developed nanoengineered lipid-polymer hybrid nanoparticles with chitosan encapsulating the steroidal antibiotic fusidic acid to evaluate its drug release and permeation, antibacterial activity, cytotoxicity, and skin safety profile for the management of wound infections. The developed nanocarriers offered sustained drug release and enhanced drug permeation; a safe profile on HaCat cell lines, suggesting non toxicity; and a reduction of five-times and four-times its inhibitory concentration was observed against MRSA 33,591 and methicillin-susceptible S. aureus (MSSA) 25921.
Microalgae Spirulina platensis is recognized as a source of lipid and polyunsaturated fatty acids, and free fatty acids were isolated to explore their antimicrobial properties for skin diseases, focusing on Candida species biofilm related to chronic wounds [47][90]. The lipid extracts were vectorized using a macroalgal-alginate nanocarrier and exhibited a good anti-biofilm activity (about 50% inhibition after 24 h at 0.1 mg/mL), and the safe application of keratinocytes, suggesting compatibility with a topical use. When enriched with spirulina, the anti-biofilm activity of lipid extracts was potentiated at low concentrations (about 80% inhibition after 24 h at 0.2 mg/mL), representing a new approach against microbial biofilm formation on chronic wounds. Thus, lipids represent a potent strategy for producing topical formulations to treat infected wounds.
3. Carbohydrates
The wide variety in the composition, structure, and function of naturally occurring carbohydrates and their derivatives amplifies the physical, chemical, and biological properties interesting in wound treatments, such as biocompatibility, bioadhesiveness, antibacterial potential, injectability, and drug delivery [48][91]. For example, chitosan is a non-toxic, antioxidant, and antimicrobial polysaccharide that has been reported to promote healing effects on infected wounds [49][92]. A study evaluated the effects of the topical co-administration of chitosan and platelet-rich plasma (PRP) on the infected burn wounds model by C. albicans in Wistar rats. Experimental groups were organized to receive any agent (control), clotrimazole (clotrimazole group), PRP (PRP group), and chitosan + PRP (chitosan + PRP group), and the wound healing processes were evaluated. The chitosan + PRP group showed a higher decrease in the wound size when compared with other groups, and antioxidant activity was improved in all the treated groups compared with the control group. It suggests a promising role of the topical application of chitosan and PRP in the infected burn wounds [50][93].
Another example is an exopolysaccharide (EPS-S3) isolated from a marine bacterium Pantoea sp. YU16-S3, investigated for its wound healing properties [51][94]. EPS-S3 showed biocompatibility with dermal fibroblasts and keratinocytes, and promoted cell adhesion, cell proliferation, and cell migration in vitro, while in vivo the exopolysaccharide induced the re-epithelializarion of injured tissue in rats, demonstrating its potential for cutaneous wound healing applications. The potential use of exopolysaccharide (EPS) produced by Nostoc sp. strains PCC7936 and PCC7413 in wound healing was evaluated by Alvarez et al. [52][95]. Both EPS may form biocompatible hydrogels and promote fibroblast migration and proliferation, and are attractive for the application in skin formulations to treat injuries.
Typical modifications such as polymerization, etherification, oxidation, crosslinking, and graft copolymerization have been explored to develop modified carbohydrates, especially polysaccharides, and have been potential candidates for wound care. Polysaccharides-based hydrogels and nanocomposites (Figure 25) show a three-dimensional network and other beneficial properties of interest for the delivery of different formulations for oral and topical applications [53][96].
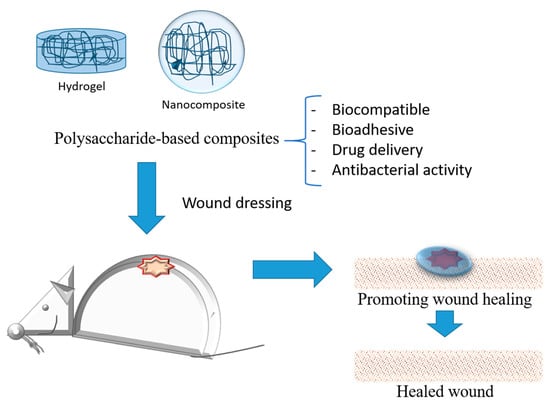
Figure 25.
Schematic representation of polysaccharides-based composites applied as wound dressing for treatment.
However, a huge challenge worldwide is to treat infected wounds, mainly non-healing chronic wounds, encouraging the use of natural polymers such as carbohydrates to create new formulations for the treatment of skin infections. In the pharmaceutical area, polysaccharide-based biomaterials have promoted controlled release and adhesion, mechanical protection, cytocompatibility, and antibacterial action, all properties of interest for infected wound healing [54][97]. Archana et al. [55][98] developed a nanocomposite based on chitosan, poly(N-vinylpyrrolidone) (PVP), and titanium dioxide (TiO2) nanoparticles that demonstrated excellent antimicrobial efficacy against pathogenic bacteria and good biocompatibility, adding to an acceleration of the healing process of open excision type wounds in albino rat models, with reductions in the open wound area from day 3. Another study reported the development of porous sponge dressings based on water-soluble thymine-modified chitosan (TC) derivatives with degrees of substitution ranging from 0.23 to 0.62 [56][99]. TC derivatives showed broad-spectrum antibacterial activities against gram-negative bacteria, gram-positive bacteria, fungi, drug-resistance bacteria, P. aeruginosa, and Acinetobacter baumannii. TC sponge dressings promoted the faster regeneration of epithelial tissue, collagen deposition, and new blood vessel formation speed, promoting the wound healing process.
Laser burn wound healing and the anti-inflammatory activity of a polysaccharide preparation from Pimpinella anisum seeds (PAP) were investigated using a carrageenan-induced paw edema model in mice [57][100]. PAP exhibited significant antioxidant, anti-inflammatory, and antibacterial activities, evidenced by a reduction on edema, cellular infiltration, and oxidative stress markers. A gel based on PAP was also developed and topically applied on laser burn lesions, and induced wound contraction, re-epithelization, and remodeling phases after seven days of treatment, suggesting that PAP is a potent natural agent for wound healing applications.
Catanzano et al. [58][101] proposed polysaccharide hydrogels combining hyaluronan (HA) in a physically cross-linked alginate (ALG) for dermal wound repair that promoted wound closure after 5 days on a rat model of an excised wound. Abourehab et al. [59][102] reported the attractive properties of alginate for wound care, such as biocompatibility and high-water absorption, minimizing bacterial contamination and wound secretions. HA also contribute to the management of wounds, regulating tissue hydrodynamics, cell migration and proliferation, and the remodeling and repair process of the extracellular matrix [60][103]. A sodium alginate-chitosan oligosaccharide-zinc oxide (SA-COS-ZnO) composite hydrogel was fabricated and evaluated for wound healing [61][104]. The hydrogel showed biocompatibility to blood cells, 3T3 cells, and 293T cells, and antibacterial activity against E. coli, S. aureus, C. albicans, and Bacillus subtilis, suggesting a promising approach to manage wound care. Dragostin et al. [62][105] developed a new chitosan-sulfonamide derivative membrane as a wound dressing biomaterial to test an in vivo study on burn wound models induced in Wistar rats. An improved healing effect with enhanced epithelialization was observed when compared with neat chitosan. Tang et al. [54][97] developed a tissue-bonded hydrogel based on the incorporation of chitosan, alginate, and polyacrylamide that demonstrated an excellent tissue adhesion, and good mechanical, biocompatibility, and antibacterial properties in the presence of E. coli and S. aureus, mainly in hydrogels containing chitosan. It suggested that positive-charged amino groups of chitosan damage the bacterial wall by electrostatic interaction with the cytoderm, leading to the release of intracellular fluids [63][106]. Hydrogels prepared through mixing cellulose and its derivatives with methacrylated gelatin also improved cell adhesion and proliferation for wound healing, favoring wound closure and accelerating wound healing [64][107]. Considering the studies reporting carbohydrates and their derivatives alone or associated with other biomaterials, free or as a composite, they represent a potent alternative to managing wound care, including burn and infected wounds.
