Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Wei Li.
Peptides, functional nutrients with a size between those of large proteins and small amino acids, are easily absorbed by the human body. Therefore, they are seeing increasing use in clinical medicine and have revealed immunomodulatory and anti-inflammatory properties which could make them effective in healing skin wounds.
- peptides
- skin wounds
- extraction
- modification
- synthesis
1. Introduction
With the development and renewal of science and technology, researchers eventually discovered a class of organic compounds whose molecular weight lies between proteins and amino acids. These compounds are easily absorbed, require low energy consumption to produce, and demonstrate high affinity, specificity, and low toxicity [1]. These compounds are known as peptides, and have been revealed as new components of therapeutic drugs. An increasing number of studies have proved that peptides have unique efficacy in antibacterial, anti-inflammatory, and anti-tumor aspects [2,3][2][3]. Given their attractive pharmacological and intrinsic properties, peptides are considered an excellent starting point for the design of new therapies, with good safety, tolerability, and efficacy in clinical application [4]. This provides huge advantages over traditional small molecules. In addition, peptide-based therapies typically have a lower production complexity than protein-based biopharmaceuticals [5], which significantly reduces production costs. Therefore, in this regard, peptides are optimally positioned between small molecules and biopharmaceuticals, and given their increased use, suitable methods for efficiently extracting them from natural sources have become the focus of attention [6]. However, many studies have shown that naturally occurring peptides are generally not suitable for direct clinical application because of their inherent weaknesses [7], including poor chemical and physical stability, and short circulating plasma half-life [8]. To address these issues, researchers must conduct studies to improve the application of peptides derived from modification and synthesis.
Although it is not fatal, skin damage often increases pain and affects the self-image of the patient; regeneration and wound healing are also essential for tissue homeostasis and the survival of organisms [9]. The causes of skin wounding are diverse, and the underlying mechanisms of wound healing are equally complex, such as inflammation [10] and oxidative stress [11]. It is well known that increasing numbers of scholars are interested in the exploration of skin diseases. Peptides have revealed many biological functions, most notably as signaling/regulatory molecules in a variety of physiological processes, including anti-inflammatory, defense, immunity, and homeostasis. These have been identified as good choices for skin healing agents [12].
2. Extraction of Peptides
In recent years, much attention has focused on the extraction and purification of peptides. Figure 1 shows the current basic process for obtaining peptides. The development and utilization of peptides also provide new ideas for the innovation of therapeutic drugs. To increase the peptide extraction rate, enzymolysis and pretreatment are often used before extraction and separation. There are many types of proteases in nature. Proteases can be divided into three categories according to their origin: proteases of plant origin, proteases of animal origin, and proteases of microbial origin. Papain is a highly active endo-cysteine protease from papaya. It is one of the widely used proteases of plant origin. Trypsin is an important endoprotease in human and animal intestines. In the pancreas, trypsin is produced by activating trypsinogen [13]. Flavourzyme is sold as an industrial peptide enzyme preparation derived from Aspergillus oryzae [14]. Proteases can also be classified according to their pH value as alkaline proteases, neutral proteases, or acidic proteases. Although all three of these proteases are found in plants and animals, microbial populations are their most widespread source [15]. Researchers have generally applied five kinds of hydrolase (Flavourzyme, trypsin, acid protease, neutral protease, and alkaline protease) to extract antioxidant peptides from the mackerel (Scomberomorus niphoniusis) defatted visceral powder. The diphenyl bitter hydrazine radical scavenging rate, hydroxyl radical scavenging rate, and hydrolysis degree are used as indicators for the selection and optimization of hydrolytic enzymes to optimize the best hydrolysis solution [16]. This was the case with apricot kernel (Semen Armeniacae Amarum) hydrolysate that was obtained by hydrolysis and degreasing with the compound protease of alkaline protease and Flavourzyme [17]. Some studies have used trypsin, Flavourzyme, and neutral and alkaline protease to extract antioxidant proteins from frog breast oil (Ranae Oviductus) [18].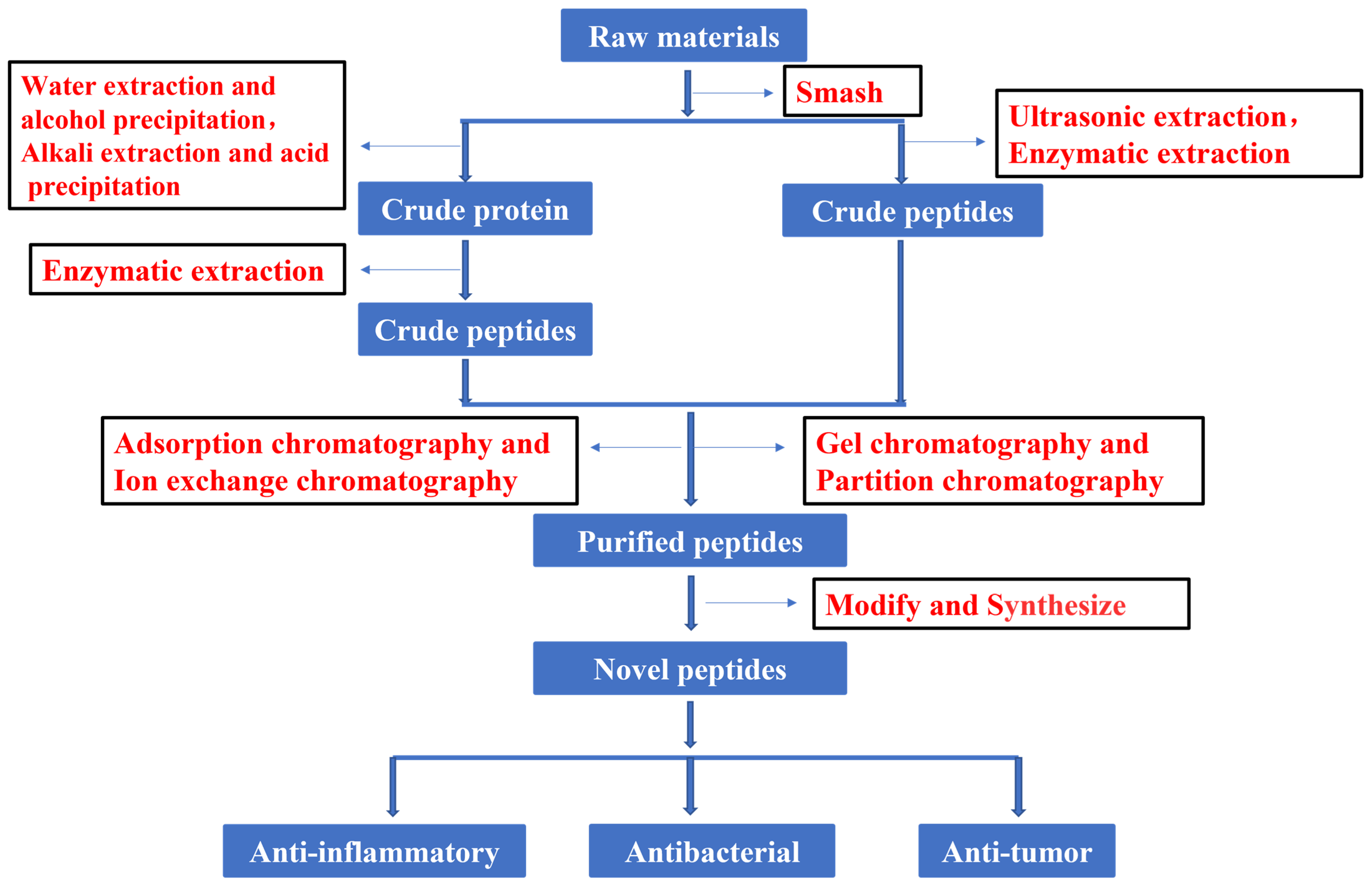
Figure 1.
Flow chart of peptide process.
3. Modification of Peptides
Natural active peptides are known to play an irreplaceable role in immune regulation [31,32][26][27], immune hormones [33][28], enzyme inhibition [34][29], and antiviral properties [35,36][30][31]. Despite their potential use as therapeutic agents, there are many potential problems with natural peptides due to their low stability and proteolysis, resulting in short activity duration and low bioavailability in vivo. One way to overcome these shortcomings is to use modified peptides, known as peptides mimics [37][32]. For example, natural peptides found in venoms could be used directly in routine therapy, but many of these peptides might need to be truncated or stabilized to improve their therapeutic properties. Thus, a complementary strategy is the generation of peptides mimics by displaying key residues forming the pharmacophore of the peptide toxin on a non-peptide scaffold [38][33]. Some studies have proposed a chemical modification box for peptides, which was used for the modification of peptides’ skeleton, amino acid side chain, and higher-order structure. This method was used to overcome the main issues encountered during the transition from natural peptides to peptide therapeutic agents, therefore promoting the synthesis and development of solid-phase peptides [39][34]. To improve the activity and increase the function of peptides, the NMEGylation-covalent binding of oligo-N-methoxyethylglycine (NMEG) chains was evaluated as a novel form of peptide/protein modification, especially for the stability and solubility of C20 peptides [40][35]. In addition, a new type of peptide was designed by a modified method, which greatly broadened the application space of peptides in different fields. To form a novel peptide, a six-membered carbon ring with an amino group on the ring binds substituted amino acids to arginine-rich peptides. Further studies found the value of this peptide in the development of cell-penetrating peptides [41][36]. The physicochemical properties of peptides are generally regulated by introducing one or more methyl groups into peptidyl amide bonds, while the pharmacokinetic properties of peptides are endowed with unprecedented characteristics [42][37].4. Synthesis of Peptides
The applications of different modification methods have significantly improved the inherent shortcomings of natural peptides, such as stability and cell penetration. In addition to designing new peptides by modification, it was possible to understand the synthesis of new compounds that do not exist in Nature by using different methods and means. Previous studies provided new ideas for the development and utilization of peptides, as well as new therapeutic directions for clinical application. In one work it was reported that a peptide was synthesized based on a known chemical formula. The basic peptide components of the Lactobacillus casei peptidoglycan complex were used as a reference to compose this chemical formula, which has potential as an effective anti-tumor agent [43][38]. A new method has been developed in which lysine residues are linked to the C-terminal of the desired peptides by a standard peptide bond during synthesis. The immobilized carboxypeptidase B (CPB) is then used to remove these lysine residues after purification, thus improving the total synthesis and purification yield of the peptides [44][39]. Similarly, there is a method in which the heterozygous organic peptides’ macrocyclic compounds are synthesized by cyclizing ribosomal-derived peptide sequences with non-peptide organic connectors [45][40]. Furthermore, cyclic RGD peptides could be efficiently synthesized based on microflow triphosgene-mediated peptide chain extension and microflow photochemical macrocyclic lactamization [46][41]. A novel strategy was also described for the generation of bicyclic peptides containing non-peptide skeleton elements, starting from recombinant peptide precursors. These compounds were produced by a ‘one-pot and two-step’ sequence in which the peptides were macrocycled via bifunctional oxyamine/1,3-amino-thiol synthetic precursors, and then the intramolecular disulfide was formed between the synthetic precursor mercaptan and a cysteine embedded in the peptide sequence [47][42]. In another one-pot method, goadsporin (GS) was synthesized using recombinant enzymes in a flexible in vitro translation system (called the FIT-GS system) [48][43].5. Beneficial Effects of Peptides on Skin
The skin is composed of epidermis, dermis and subcutaneous tissue. Understanding skin structure is fundamental for the treatment of all skin conditions. The healing of skin wounds is an important biological process which can regenerate new skin after a wound. Skin injuries can be divided into skin trauma and burns, skin disease, and skin cancer. Among them, chronic wounds caused by skin injuries and burns are the most common skin diseases due to the slow healing of hypoxia, abnormal peripheral sensory nerve function, and insufficient blood tissue supply. The most significant sign of chronic wounds is severe abnormal immune skin function [49][44]. The active components of peptides could serve as first-line innate immune defense against exogenous microorganisms in the skin, in addition to coordinating adaptive immune responses to perform various immunomodulatory functions. Different authors found that peptides repair skin damage through a variety of mechanisms (Figure 2) [50,51][45][46]. Many skin diseases and injuries have been reported to involve the production of ROS radicals [52][47], and a dramatic increase in ROS levels can cause oxidative stress. Peptides acting on the skin can have a therapeutic effect by inhibiting the production of ROS. In addition, the skin has a vast antioxidant system, including superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT) [53][48], and the therapeutic process of peptides on the skin involves the regulation of these factors. When skin pathology occurs, it is often regulated by the PI3K/AKT [54][49], MAPK/ERK [55][50], and TGFβ/Smad pathways [56][51]. Further studies have shown that peptides can regulate inflammatory factors (IL-1, IL-6, IL-8) or matrix metalloproteinases (MMP1, MMP2, MMP3) by PI3K/AKT, MAPK/ERK, and TGFβ/Smad pathways, thereby reducing the inflammatory response of the skin [57,58][52][53].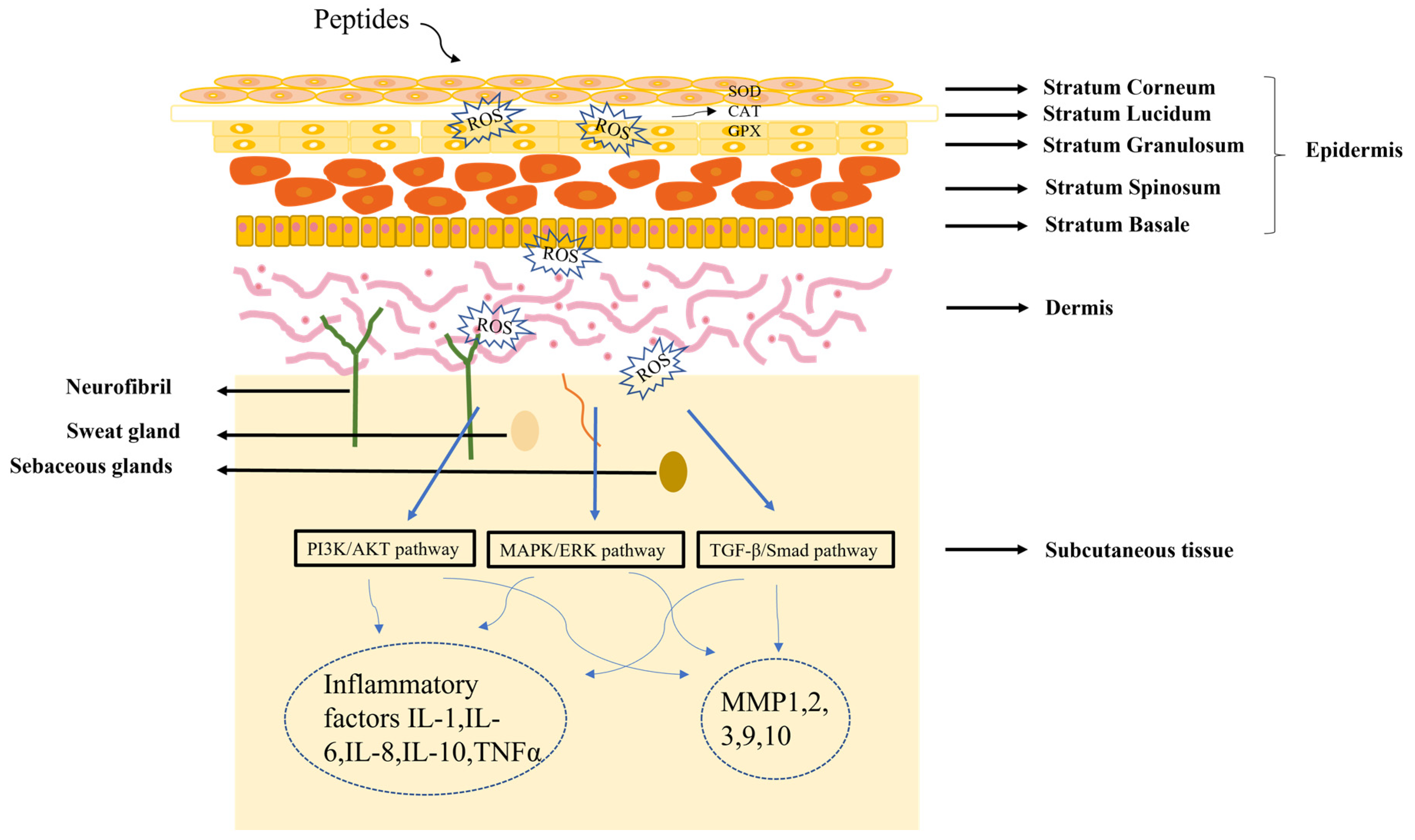
Figure 2.
Diagram of the mechanism of peptide treatment of skin damage.
References
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16.
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128.
- Yavari, B.; Mahjub, R.; Saidijam, M.; Raigani, M.; Soleimani, M. The Potential Use of Peptides in Cancer Treatment. Curr. Protein Pept. Sci. 2018, 19, 759–770.
- Agyei, D.; Ahmed, I.; Akram, Z.; Iqbal, H.M.N.; Danquah, M.K. Protein and Peptide Biopharmaceuticals: An Overview. Protein Pept. Lett. 2017, 24, 94–101.
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707.
- Borrajo, P.; Pateiro, M.; Barba, F.J.; Mora, L.; Franco, D.; Toldra, F.; Lorenzo, J.M. Antioxidant and Antimicrobial Activity of Peptides Extracted from Meat By-products: A Review. Food Anal. Method 2019, 12, 2401–2415.
- Sarker, A. A review on the application of bioactive peptides as preservatives and functional ingredients in food model systems. J. Food Process. Preserv. 2022, 46, e16800.
- Acquah, C.; Chan, Y.W.; Pan, S.; Agyei, D.; Udenigwe, C.C. Structure-informed separation of bioactive peptides. J. Food Biochem. 2019, 43, e12765.
- Chin, J.S.; Madden, L.; Chew, S.Y.; Becker, D.L. Drug therapies and delivery mechanisms to treat perturbed skin wound healing. Adv. Drug Deliver. Rev. 2019, 149, 2–18.
- Han, Y.P.; Tuan, T.L.; Wu, H.; Hughes, M.; Garner, W.L. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J. Cell Sci. 2001, 114, 131–139.
- Lee, J.H.; Lim, J.Y.; Jo, E.H.; Noh, H.M.; Park, S.; Park, M.C.; Kim, D.K. Chijabyukpi-Tang Inhibits Pro-Inflammatory Cytokines and Chemokines via the Nrf2/HO-1 Signaling Pathway in TNF-alpha/IFN-gamma-Stimulated HaCaT Cells and Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice. Front. Pharmacol. 2020, 11, 1018.
- de Souza, G.S.; de Jesus Sonego, L.; Santos Mundim, A.C.; de Miranda Moraes, J.; Sales-Campos, H.; Lorenzon, E.N. Antimicrobial-wound healing peptides: Dual-function molecules for the treatment of skin injuries. Peptides 2022, 148, 170707.
- Walmsley, S.J.; Rudnick, P.A.; Liang, Y.; Dong, Q.; Stein, S.E.; Nesvizhskii, A.I. Comprehensive analysis of protein digestion using six trypsins reveals the origin of trypsin as a significant source of variability in proteomics. J. Proteome Res. 2013, 12, 5666–5680.
- Merz, M.; Eisele, T.; Berends, P.; Appel, D.; Rabe, S.; Blank, I.; Stressler, T.; Fischer, L. Flavourzyme, an Enzyme Preparation with Industrial Relevance: Automated Nine-Step Purification and Partial Characterization of Eight Enzymes. J. Agric Food Chem. 2015, 63, 5682–5693.
- Gurumallesh, P.; Alagu, K.; Ramakrishnan, B.; Muthusamy, S. A systematic reconsideration on proteases. Int. J. Biol. Macromol. 2019, 128, 254–267.
- Xue, Y.; Guo, R.; Zhang, B. . Se Pu 2020, 38, 1431–1439.
- Zhang, H.; Xue, J.; Zhao, H.; Zhao, X.; Xue, H.; Sun, Y.; Xue, W. Isolation and Structural Characterization of Antioxidant Peptides from Degreased Apricot Seed Kernels. J AOAC Int. 2018, 101, 1661–1663.
- Wang, S.; Gan, Y.; Mao, X.; Kan, H.; Li, N.; Zhang, C.; Wang, Z.; Wang, Y. Antioxidant Activity Evaluation of Oviductus Ranae Protein Hydrolyzed by Different Proteases. Molecules 2021, 26, 1625.
- Chang, C.H.; Chang, H.Y.; Rappsilber, J.; Ishihama, Y. Isolation of Acetylated and Unmodified Protein N-Terminal Peptides by Strong Cation Exchange Chromatographic Separation of TrypN-Digested Peptides. Mol. Cell. Proteom. 2021, 20, 100003.
- Jahandideh, F.; Liu, P.; Wu, J. Purification and identification of adipogenic-differentiating peptides from egg white hydrolysate. Food Chem. 2018, 259, 25–30.
- Wan, M.Y.; Dong, G.; Yang, B.Q.; Feng, H. Identification and characterization of a novel antioxidant peptide from feather keratin hydrolysate. Biotechnol. Lett. 2016, 38, 643–649.
- Hu, B.; Xu, L.; Li, Y.; Bai, X.; Xing, M.; Cao, Q.; Liang, H.; Song, S.; Ji, A. A peptide inhibitor of macrophage migration in atherosclerosis purified from the leech Whitmania pigra. J. Ethnopharmacol. 2020, 254, 112723.
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016, 204, 365–372.
- Zhang, Y.; Gao, X.C.; Pan, D.D.; Zhang, Z.G.; Zhou, T.Q.; Dang, Y.L. Isolation, characterization and molecular docking of novel umami and umami-enhancing peptides from Ruditapes philippinarum. Food Chem. 2021, 343, 128522.
- Joshi, I.; Nazeer, R.A. EGLLGDVF: A Novel Peptide from Green Mussel Perna viridis Foot Exerts Stability and Anti-Inflammatory Effects on LPS-Stimulated RAW264.7 Cells. Protein Pept. Lett. 2020, 27, 851–859.
- Villegas-Escobar, V.; Ceballos, I.; Mira, J.J.; Argel, L.E.; Peralta, S.O.; Romero-Tabarez, M. Fengycin C Produced by Bacillus subtilis EA-CB0015. J. Nat. Prod. 2013, 76, 503–509.
- Schwardt, O.; Lamers, C.; Bechtler, C.; Ricklin, D. Therapeutic Peptides as Emerging Options to Restore Misguided Host Defence and Homeostasis: From Teaching to Concept to Clinic. Chimia 2021, 75, 495–499.
- Pfeil, J.; Simonetti, M.; Lauer, U.; Volkmer, R.; von Thulen, B.; Durek, P.; Krahmer, R.; Leenders, F.; Hamann, A.; Hoffmann, U. Tolerogenic Immunomodulation by PEGylated Antigenic Peptides. Front. Immunol. 2020, 11, 529035.
- Ambadapadi, S.; Munuswamy-Ramanujam, G.; Zheng, D.H.; Sullivan, C.; Dai, E.; Morshed, S.; McFadden, B.; Feldman, E.; Pinard, M.; McKenna, R.; et al. Reactive Center Loop (RCL) Peptides Derived from Serpins Display Independent Coagulation and Immune Modulating Activities. J. Biol. Chem. 2016, 291, 2874–2887.
- Zhao, B.L.; Su, K.Y.; Mao, X.L.; Zhang, X.W. Separation and identification of enzyme inhibition peptides from dark tea protein. Bioorganic Chem. 2020, 99, 103772.
- Nyanguile, O. Peptide Antiviral Strategies as an Alternative to Treat Lower Respiratory Viral Infections. Front. Immunol. 2019, 10, 1366.
- Conlon, J.M.; Mechkarska, M.; Lukic, M.L.; Flatt, P.R. Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides 2014, 57, 67–77.
- Gentilucci, L.; De Marco, R.; Cerisoli, L. Chemical Modifications Designed to Improve Peptide Stability: Incorporation of Non-Natural Amino Acids, Pseudo-Peptide Bonds, and Cyclization. Curr Pharm Design 2010, 16, 3185–3203.
- Brady, R.M.; Baell, J.B.; Norton, R.S. Strategies for the Development of Conotoxins as New Therapeutic Leads. Mar Drugs 2013, 11, 2293–2313.
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide chemistry toolbox—Transforming natural peptides into peptide therapeutics. Bioorganic Med. Chem. 2018, 26, 2759–2765.
- Park, M.; Jardetzky, T.S.; Barron, A.E. NMEGylation: A Novel Modification to Enhance the Bioavailability of Therapeutic Peptides. Biopolymers 2011, 96, 688–693.
- Kato, T.; Kita, Y.; Iwanari, K.; Asano, A.; Oba, M.; Tanaka, M.; Doi, M. Synthesis of six-membered carbocyclic ring alpha, alpha-disubstituted amino acids and arginine-rich peptides to investigate the effect of ring size on the properties of the peptide. Bioorganic Med. Chem. 2021, 38, 116111.
- Chatterjee, J.; Laufer, B.; Kessler, H. Synthesis of N-methylated cyclic peptides. Nat. Protoc. 2012, 7, 432–444.
- Fichera, G.A.; Fichera, M.; Milone, G. Antitumoural activity of a cytotoxic peptide of Lactobacillus casei peptidoglycan and its interaction with mitochondrial-bound hexokinase. Anticancer Drugs 2016, 27, 609–619.
- Chemuru, S.; Kodali, R.; Wetzel, R. Improved chemical synthesis of hydrophobic Abeta peptides using addition of C-terminal lysines later removed by carboxypeptidase B. Biopolymers 2014, 102, 206–221.
- Smith, J.M.; Fasan, R. Synthesis of macrocyclic organo-peptide hybrids from ribosomal polypeptide precursors via CuAAC-/hydrazide-mediated cyclization. Methods Mol. Biol. 2015, 1248, 23–38.
- Elsawy, M.A.; Tikhonova, I.G.; Martin, L.; Walker, B. Smac-Derived Aza-Peptide as an Aminopeptidase-Resistant XIAP BIR3 Antagonist. Protein Pept. Lett. 2015, 22, 836–843.
- Smith, J.M.; Hill, N.C.; Krasniak, P.J.; Fasan, R. Synthesis of bicyclic organo-peptide hybrids via oxime/intein-mediated macrocyclization followed by disulfide bond formation. Org. Biomol. Chem. 2014, 12, 1135–1142.
- Ozaki, T.; Yamashita, K.; Goto, Y.; Shimomura, M.; Hayashi, S.; Asamizu, S.; Sugai, Y.; Ikeda, H.; Suga, H.; Onaka, H. Dissection of goadsporin biosynthesis by in vitro reconstitution leading to designer analogues expressed in vivo. Nat. Commun. 2017, 8, 14207.
- Petkovic, M.; Mouritzen, M.V.; Mojsoska, B.; Jenssen, H. Immunomodulatory Properties of Host Defence Peptides in Skin Wound Healing. Biomolecules 2021, 11, 952.
- Norlen, L.; Lundborg, M.; Wennberg, C.; Narangifard, A.; Daneholt, B. The Skin’s Barrier: A Cryo-EM Based Overview of its Architecture and Stepwise Formation. J Invest Dermatol 2022, 142, 285–292.
- Mansfield, K.; Naik, S. Unraveling Immune-Epithelial Interactions in Skin Homeostasis and Injury. Yale J. Biol. Med. 2020, 93, 133–143.
- Awad, F.; Assrawi, E.; Louvrier, C.; Jumeau, C.; Giurgea, I.; Amselem, S.; Karabina, S.A. Photoaging and skin cancer: Is the inflammasome the missing link? Mech. Ageing Dev. 2018, 172, 131–137.
- Baek, J.; Lee, M.G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016, 21, 164–169.
- Teng, Y.; Fan, Y.; Ma, J.; Lu, W.; Liu, N.; Chen, Y.; Pan, W.; Tao, X. The PI3K/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders. Cells 2021, 10, 1219.
- Lugović-Mihić, L.; Ćesić, D.; Vuković, P.; Novak Bilić, G.; Šitum, M.; Špoljar, S. Melanoma Development: Current Knowledge on Melanoma Pathogenesis. Acta Dermatovenerol. Croat. 2019, 27, 163–168.
- Kasuya, A.; Tokura, Y. Attempts to accelerate wound healing. J. Dermatol. Sci. 2014, 76, 169–172.
- Bang, J.S.; Jin, Y.J.; Choung, S.Y. Low molecular polypeptide from oyster hydrolysate recovers photoaging in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2020, 386, 114844.
More
