Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 4 by Camila Xu.
Major mechanisms of Bifidobacterium action include modulation of adaptive and innate immunity, enhancement of intestinal epithelial barrier, prevention of pathogen adhesion, and production of antimicrobial compounds.
- gut microbiota
- Bifidobacterium
- probiotic
- preterm infants
1. Bifidobacterium: Mechanism of Action
Probiotic strains can modulate the host immune system through several mechanisms (Figure 1). Major mechanisms of action include modulation of adaptive and innate immunity, enhancement of intestinal epithelial barrier, prevention of pathogen adhesion, and production of antimicrobial compounds, which have been discussed in detail as follows.
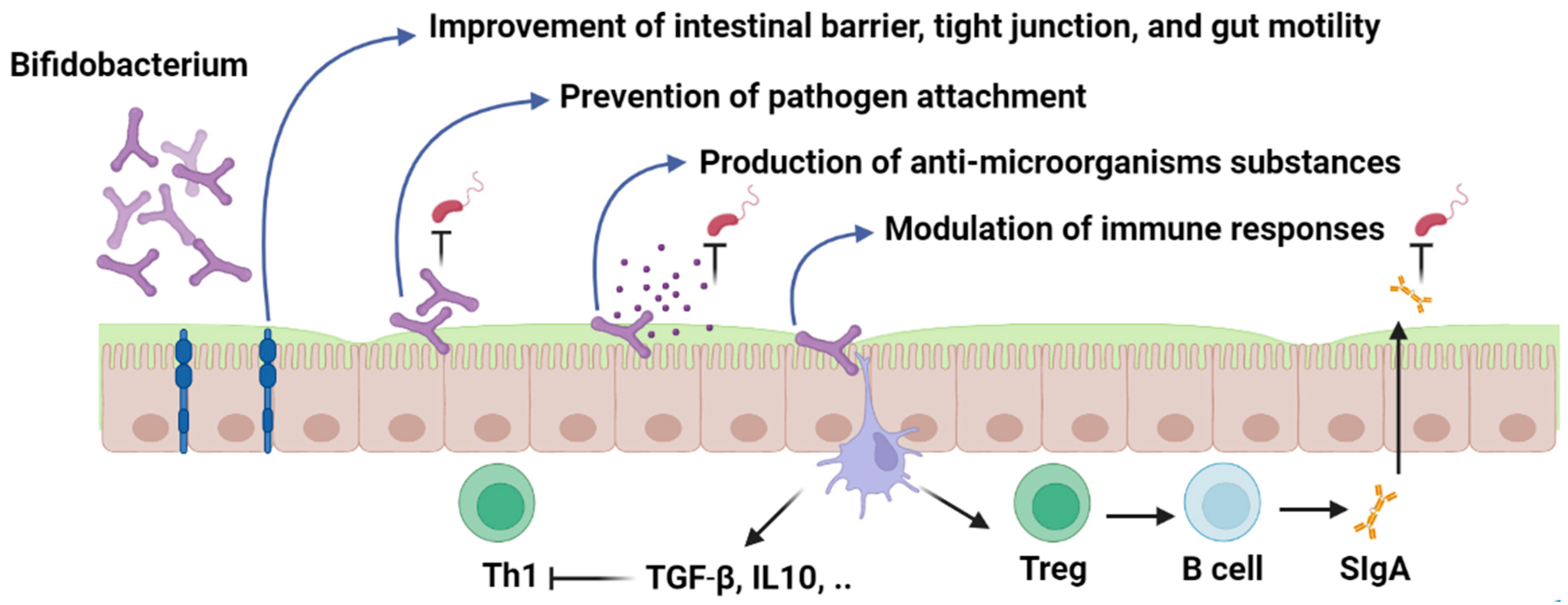
Figure 1. Bifidobacterium; mechanism of action. Figures in this manuscript were created specifically for this manuscript in BioRender.com, accessed on 15 August 2022.
2. Modulation of the Immune System
The innate immune system also known as the nonspecific immune system is the first line of defense in the human body including the protective effects of skin and mucosal membrane and immune system cells. While the adaptive immune system is a specific immunity to identifying pathogens by specialized immune cells including B and T lymphocyte cells [1]. Probiotics can modulate innate and adaptive immunity and lead to the enhancement of intestinal epithelium through immune mediators such as Toll-like receptors (TLRs), cytosolic signaling receptors such as nucleotide-binding oligomerization domain leucine-rich repeat-containing and pyrin domain-containing (NLRP), and anti-inflammatory cytokines.
3. Intracellular Immune Receptors (TLRs, NLRs) and Anti-Inflammatory Mediators
Intracellular immune receptors have a remarkable role in recognizing pathogen-associated molecular patterns (PAMPs) and microbial signals. Toll-like receptors (TLRs) are highly expressed in immune cells (dendritic cells, macrophages, and Natural killer cells (NK)) and non-immune cells (endothelial and epithelial cells). Recognition of microbial compounds by TLRs leads to the activation of downstream immune responses and the production of several inflammatory cytokines and other immune mediators which lead to innate and adaptive immune responses [2]. Enterocytes or intestinal absorptive cells line the inner surface of the intestine and express TLR4 as abundant proteins on their outer surface which are in close contact with microbial compounds in the gut lumen. TLR4 can recognize Lipopolysaccharide (LPS) in Gram-negative bacteria and activate MYD88 protein (myeloid differentiation primary response 88). Activation of MYD88 leads to kinase activation and degradation of NFκB/IKB dimer (Nuclear factor kappa-light-chain-enhancer of activated B cells)/(an inhibitory protein bound to NFκB). After the degradation of the NF-κB/IKB dimer, NFκB complex is translocated to the nucleus where the gene transcription of many pro-inflammatory cytokines, tumor necrosis factor-alpha (TNFα), and interleukin occur [3]. As previously mentioned, TLR4 stimulation by Gram-negative bacteria causes enterocyte death and mucosal injury, both of which have been related to the etiology of NEC in several studies.
It has been shown that Bifidobacterium probiotics and their metabolites can alter the transcriptional activity of enterocytes and modulate the intestinal innate immune response. For instance, probiotic-conditioned media (PCM) with a single probiotic strain or combined probiotic strains including B. infantis and L. acidophilus could lead to a significant decrease in the expression of IL-1β, IL-8, IL-6, TLR2 mRNA, and TLR4 mRNA and high expression of inflammatory inhibitors (Tollip and SIGIRR). Exposure of PCM with primary enterocyte cultures of NEC tissue has also led to down-regulation of IL-6, IL-8, and TLR2 and up-regulation of Tollip and SIGIRR [4].
Similar to this, transcription profiling of immature human fetal intestinal epithelial cells exposed to B. infantis and L. acidophilus revealed modification of several genes involved in immune responses and cell survival pathways. Probiotic conditioned media (PCM)-exposed cells displayed decreased NF-B pathway gene expression as well as IL-6 and IL-8 levels. As a result of PCM exposure, genes involved in remodeling the extracellular matrix were also downregulated [5]. Given the strong influence of probiotic strains on the regulation of NF-κB pathways, it can be a potential therapeutic strategy to manipulate receptors and cytokines which leads to the activation of this pathway in the functionally immature intestinal tract in preterm infants. TLR2 detected on the surface of several immune cells has also the same function as TLR4. Since immature enterocytes in preterm infants have been associated with high expression of TLR2, probiotic administrations have shown a significant impact on the regulation of TLR2-ligand interaction. The heterodimeric complex of TLR2 with TLR1 and TLR6 can recognize Gram-positive bacteria compounds such as lipoteichoic acids, peptidoglycan, and lipopeptides. The interaction of TLRs and microbial signals leads to the activation of a cascade of immune responses [6].
Modulation of TLR2 and TLR4 expression and development of the immune system through probiotic strain activities have been investigated in several animal-based studies. For instance, an investigation of Bifidobacterium administration in intestinal epithelial cells in rat models showed that TLR2 expression was significantly lower in intestinal epithelial cells treated with different strains of Bifidobacterium (B. longum, B. infantis, and B. youth). While cells infected by E. coli endotoxin showed higher expression of TLR2 and TLR4. Also, intestinal barrier function measured by transepithelial/transendothelial electrical resistance (TEER) was significantly higher in Bifidobacterium-treated cells compared to cells infected by E. coli endotoxin [7].
Human cytokine synthesis inhibitory factor (CSIF) or interleukin 10 (IL-10) mainly produced by monocytes and other immune cells such as Th2, Treg, mast cells, and B cells, is another anti-inflammatory cytokine that can be regulated by probiotics. IL-10 can suppress the production of several pro-inflammatory cytokines including TNFα, IFN-γ, GM-CSF, IL-2, and IL-3 [8]. Animal-based studies have also confirmed the regulatory effect of Bifidobacterium strains on IL-10 and subsequently the prevention of inflammatory bowel diseases. For instance, L. casei and B. breve-treated mouse models could selectively enhance the amount of IL-10-producing CD4+ T cells in the large intestine by twofold without altering intestinal microbiota [9]. B. adolescentis supplementation in preterm rat models could also decrease the development of NEC through the modulation of inhibitory adaptor proteins such as TOLLIP, and inhibitory receptor toll interleukin-1R 8 (SIGIRR) and expression of TLR4 [10].
Inhibition of NLRP3 inflammasome (NOD-, LRR- and pyrin domain-containing protein 3) has also shown promising results in the prevention of gastrointestinal disorders. NLRP3 inflammasome is a cytosolic multiprotein oligomer in the innate immune system belonging to the nucleotide-binding oligomerization domain-like receptors (NOD-like receptors: NLRs).
NLRP3 acts as a pattern recognition receptor (PRR) and can detect microbial signals and lead to the production of proinflammatory cytokines (IL-1β) and caspase 1 [11]. Overactivation of NLRP3 has been associated with the development of different inflammatory diseases which can be regulated by the inhibitory effects of probiotic strains [12]. Investigation of NLRP3 inflammasome in NEC mouse models treated with NLRP3 inhibitor MCC950 showed that the NEC mouse model showed higher expression of NLRP3 in the intestine and brain and mature IL-1β compared to mice receiving NLRP3 inhibitor (MCC950). As a result, inflammatory cytokines, NEC survival rate, and histological damage in the brain and gut were all dramatically decreased by MCC950 treatment, demonstrating the significance of blocking the NLRP3 pathway in the prevention of inflammatory bowel disorders [8].
4. Regulation of Intestinal Epithelium Function
The gastrointestinal barrier provides a vast surface for interacting with microbial signals and environmental stimuli. This contact has a substantial impact on the host’s physiology and may trigger a regulated and normal immunological response or infection development, depending on the initial stimulus. The outermost layer of the intestinal epithelium is made up of enterocytes, Paneth cells, goblet cells, intraepithelial lymphocytes, and enteroendocrine cells. While controlling permeability and microbial translocation, epithelial tight junctions (TJs) between intestinal cells maintain the integrity of the intestinal barrier [13]. Increased intestinal permeability, TJ disruption, and subsequent uncontrolled translocation of microbial pathogens (leaky gut) may occur in preterm newborns with an underdeveloped gut barrier and lead to gastrointestinal diseases.
Human and animal trials have shown the prophylactic effects of Bifidobacterium strains on the intestinal barrier [14]. Investigation of Bifidobacterium’s role on the TJ and intestinal barrier in animal models and human intestinal cell models (Caco-2) has shown that Bifidobacterium administration can down-regulate the expression of proinflammatory cytokines and improve transepithelial electrical resistance and permeability of Caco-2. Bifidobacterium in 108 CFU could also increase the expression of ZO-1, occludin, and claudins (TJ proteins) (p < 0.01) compared to Caco-2 monolayers treated with LPS. Moreover, compared to the LPS-induced enterocyte barrier injury of Caco-2 monolayers (E. coli 055), and LPS-fed mice models, Bifidobacterium significantly suppressed the expression of TNF-α and IL-6 and decreased the NEC rate from 88 to 47% (p < 0.05) in controls [15]. Another study showed a different approach in regulation of intestinal barrier by Bifidobacterium strains. This study has demonstrated that B. bifidum (108 CFU) might improve the intestinal epithelial tight junction barrier in Caco-2 monolayers by targeting the TLR2 pathway in an NF-B-Independent manner (attachment with enterocyte TLR-2 receptors and stimulation of p38 kinase pathway) [16].
5. Competitive Exclusion and Adhesion Properties
Elimination of pathogens with identical needs for resources by probiotic strains known as competitive exclusion is a common strategy applied by probiotic microorganisms in the gastrointestinal tract [17]. Adhesion of probiotics to the intestinal epithelium can prevent the attachment and colonization of bacterial pathogens, especially enteropathogens, and resultant infections. Probiotics adhesion can also enhance host-probiotic interaction which leads to longer transient colonization time and provide sufficient time to express their immunomodulatory effects while attached to the epithelial receptors [18].
Serine protease inhibitor (serpin) produced by B. longum subsp. Longum NCC2705, B. longum subsp. Infantis, B. dentium, and B. breve and pentapeptide (CHWPR) in B. animalis are common extracellular proteins that facilitate host-probiotic interaction. Neutrophil and pancreatic elastases which are produced during inflammation by immune cells can be prevented by Bifidobacterium serin and suppress inflammatory responses and immune cell recruitment [19]. CHWPR can also pass through the cytoplasmic membrane and reach the nucleus and upregulate c-myc and il-6 genes, which are involved in many cellular metabolisms including gastrointestinal tract physiology [20].
Several in vitro and in vivo studies have investigated different extracellular proteins in probiotics using intestinal cell lines to evaluate the antagonistic interactions between pathogens and probiotics. In a study assessing the adhesion ability of 12 commonly used probiotic strains and antagonistic interactions with enteropathogens (Enterobacter, Clostridium, Staphylococcus, and Bacteroides), all tested probiotic strains could prevent bacterial pathogen colonization in the intestinal epithelium models [21]. Tight adhesion (Tad) pili (Type IVb pili) in B. breve UCC2003 has also been found to be a critical element for gut colonization (202) and has a proliferation impact on intestinal epithelial cells in mice models [22]. A comparative study on the physiological characteristics and acid-resistant phenotype of B. longum and B. catenulatum has shown that acid-resistant Bifidobacterium strains showed a greater adhesion to the human intestinal mucus and a higher displacement ability (competitive exclusion) on E. coli, Salmonella enterica serovar Typhimurium, Listeria monocytogenes, Enterobacter sakazakii, and Clostridium difficile from adhering to human intestinal mucus compared to the acid-sensitive strains. These results highlight the significance of carefully evaluating the safety, effectiveness, and phenotypic traits of probiotic strains before clinical trial research [23].
The human plasminogen-binding activity of different species of Bifidobacterium (B. bifidum, B. longum, and Bifidobacterium lactis) has also shown that Bifidobacterium has a unique adhesion ability through degradation of the extracellular matrix which allows Bifidobacterium-host interaction [24].
In another study, the phenotypic characteristics of B. breve and B. longum isolated from preterm and full-term infants were examined. This study revealed a significant variation across different isolates in terms of Caco-2 cells adhesion, surface hydrophobicity, and autoaggregation properties which may show strain-specific phenotypic traits that should be considered when choosing the probiotic candidate for modifying the gut microbiota in preterm newborns [25]. It might also explain why, despite probiotic treatment, some investigations have not shown successful competitive exclusion or fecal detection of Bifidobacterium. These findings may point to the need for a case-by-case comparison of probiotic strains and infectious agents in order to identify the optimal probiotic candidate with the potential to adhere to and colonize the gastrointestinal tract while also improving disease outcomes.
6. Synthesis of Antimicrobial Compounds
Another successful tactic against Gram-positive and Gram-negative bacteria is the production of antibacterial compounds by probiotic strains. There have been several low molecular weight compounds (LMWs) found in Bifidobacterium strains that show inhibitory properties against pathogens. For instance, short-chain fatty acids (such as acetate, butyrate, and propionate) are the end-products of the metabolism of human undigestible carbohydrates produced by gut microbiota and probiotic strains and have been used as health indicators in the diagnosis of gastrointestinal diseases. In multiple human and animal investigations, the administration of Bifidobacterium was linked to greater levels of short-chain fatty acids and a reduction in intestinal damage [26][27][28]. Numerous studies have also linked LMW lipophilic compounds to the inhibitory actions of Bifidobacterium [29][30]. In Caco-2 cells and mouse models, for example, the antibacterial activity of 14 Bifidobacterium strains isolated from newborn fecal samples against S Typhimurium SL1344 revealed antagonistic action of Bifidobacterium strains either through cell entry prevention or intracellular inhibition [29].
References
- McComb, S.; Thiriot, A.; Akache, B.; Krishnan, L.; Stark, F. Introduction to the immune system. In Immunoproteomics; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–24.
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 461.
- Dąbek, J.; Kułach, A.; Gąsior, Z. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB): A new potential therapeutic target in atherosclerosis? Pharmacol. Rep. 2010, 62, 778–783.
- Ganguli, K.; Meng, D.; Rautava, S.; Lu, L.; Walker, W.A.; Nanthakumar, N. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G132–G141.
- Guo, S.; Guo, Y.; Ergun, A.; Lu, L.; Walker, W.A.; Ganguli, K. Secreted metabolites of Bifidobacterium infantis and Lactobacillus acidophilus protect immature human enterocytes from IL-1β-induced inflammation: A transcription profiling analysis. PLoS ONE 2015, 10, e0124549.
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The role of TLR2 in infection and immunity. Front. Immunol. 2012, 3, 79.
- Yang, X.; Gao, X.-C.; Liu, J.; Ren, H.-Y. Effect of EPEC endotoxin and bifidobacteria on intestinal barrier function through modulation of toll-like receptor 2 and toll-like receptor 4 expression in intestinal epithelial cell-18. World J. Gastroenterol. 2017, 23, 4744.
- Zhu, F.; Wang, L.; Gong, Z.; Wang, Y.; Gao, Y.; Cai, W.; Wu, J. Blockage of NLRP3 inflammasome activation ameliorates acute inflammatory injury and long-term cognitive impairment induced by necrotizing enterocolitis in mice. J. Neuroinflammation 2021, 18, 66.
- Jeon, S.G.; Kayama, H.; Ueda, Y.; Takahashi, T.; Asahara, T.; Tsuji, H.; Tsuji, N.M.; Kiyono, H.; Ma, J.S.; Kusu, T. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012, 8, e1002714.
- Wu, W.; Wang, Y.; Zou, J.; Long, F.; Yan, H.; Zeng, L.; Chen, Y. Bifidobacterium adolescentis protects against necrotizing enterocolitis and upregulates TOLLIP and SIGIRR in premature neonatal rats. BMC Pediatr. 2017, 17, 1.
- JIANG, H.; YiQing, Y.; JIANG, W.; RongBin, Z. NLRP3 inflammasome: Activation, regulation, and role in diseases. Sci. Sin. Vitae 2017, 47, 125–131.
- Gomez-Lopez, N.; Romero, R.; Garcia-Flores, V.; Leng, Y.; Miller, D.; Hassan, S.S.; Hsu, C.-D.; Panaitescu, B. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes. Biol. Reprod. 2019, 100, 1306–1318.
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20.
- Hsieh, C.Y.; Osaka, T.; Moriyama, E.; Date, Y.; Kikuchi, J.; Tsuneda, S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol. Rep. 2015, 3, e12327.
- Ling, X.; Linglong, P.; Weixia, D.; Hong, W. Protective effects of bifidobacterium on intestinal barrier function in LPS-induced enterocyte barrier injury of Caco-2 monolayers and in a rat NEC model. PLoS ONE 2016, 11, e0161635.
- Al-Sadi, R.; Dharmaprakash, V.; Nighot, P.; Guo, S.; Nighot, M.; Do, T.; Ma, T.Y. Bifidobacterium bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner. Int. J. Mol. Sci. 2021, 22, 8070.
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514.
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Factories 2020, 19, 23.
- Ivanov, D.; Emonet, C.; Foata, F.; Affolter, M.; Delley, M.; Fisseha, M.; Blum-Sperisen, S.; Kochhar, S.; Arigoni, F. A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase-like serine proteases. J. Biol. Chem. 2006, 281, 17246–17252.
- Mitsuma, T.; Odajima, H.; Momiyama, Z.; Watanabe, K.; Masuguchi, M.; Sekine, T.; Shidara, S.; Hirano, S. Enhancement of gene expression by a peptide p (CHWPR) produced by Bifidobacterium lactis BB-12. Microbiol. Immunol. 2008, 52, 144–155.
- Collado, M.; Meriluoto, J.; Salminen, S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett. Appl. Microbiol. 2007, 45, 454–460.
- O’Connell Motherway, M.; Houston, A.; O’Callaghan, G.; Reunanen, J.; O’Brien, F.; O’Driscoll, T.; Casey, P.G.; de Vos, W.M.; van Sinderen, D.; Shanahan, F. A Bifidobacterial pilus-associated protein promotes colonic epithelial proliferation. Mol. Microbiol. 2019, 111, 287–301.
- Collado, M.C.; Gueimonde, M.; Sanz, Y.; Salminen, S. Adhesion properties and competitive pathogen exclusion ability of bifidobacteria with acquired acid resistance. J. Food Prot. 2006, 69, 1675–1679.
- Candela, M.; Bergmann, S.; Vici, M.; Vitali, B.; Turroni, S.; Eikmanns, B.J.; Hammerschmidt, S.; Brigidi, P. Binding of human plasminogen to Bifidobacterium. J. Bacteriol. 2007, 189, 5929–5936.
- Andriantsoanirina, V.; Teolis, A.-C.; Xin, L.X.; Butel, M.J.; Aires, J. Bifidobacterium longum and Bifidobacterium breve isolates from preterm and full term neonates: Comparison of cell surface properties. Anaerobe 2014, 28, 212–215.
- Wang, C.; Shoji, H.; Sato, H.; Nagata, S.; Ohtsuka, Y.; Shimizu, T.; Yamashiro, Y. Effects of oral administration of Bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 252–257.
- Mohan, R.; Koebnick, C.; Schildt, J.; Mueller, M.; Radke, M.; Blaut, M. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr. Res. 2008, 64, 418–422.
- Trindade, L.; Martins, V.; Rodrigues, N.; Souza, E.; Martins, F.; Costa, G.; Almeida-Leite, C.; Faria, A.; Cardoso, V.; Maioli, T. Oral administration of Simbioflora®(synbiotic) attenuates intestinal damage in a mouse model of 5-fluorouracil-induced mucositis. Benef. Microbes 2018, 9, 477–486.
- Liévin, V.; Peiffer, I.; Hudault, S.; Rochat, F.; Brassart, D.; Neeser, J.; Servin, A. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut 2000, 47, 646–652.
- Gibson, G.R.; Wang, X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 1994, 77, 412–420.
More
