Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Arastu Sharma and Version 3 by Jessie Wu.
Nicotinamide adenine dinucleotide (NAD+) is one of the primary coenzymes in metabolic processes and is involved with numerous other pathways, such as energy expenditure, metabolic and stress adaptations, and circadian rhythm maintenance. NAD+ levels sharply decline with age, and this decline can be attributed to the activity of CD38, an enzyme responsible for the degradation of NAD+, which disrupts the NAD+ synthesis pathways during the course of aging.
- aging
- longevity
- nicotinamide adenine dinucleotide
- nutraceuticals
- healthspan
- lifespan
- homeostasis
1. Introduction
Nicotinamide adenine dinucleotide (NAD+) homeostasis in the body is critical for optimal biological function. NAD+ consuming enzymes function to convert NAD+ to nicotinamide (NAM) and occupy a specific role in biological aging pathology. They are implicated as interventional targets for geroprotection, such as the enzyme CD38, the sirtuins (SIRT) deacetylases, poly [ADP-ribose] polymerase 1 (PARP1: involved in the DNA damage response (DDR)), and the neuronal degenerating and NAD+ draining factor SARM1 [1][2][18,19]. NAD+ can be synthesized de novo from nicotinic acid (NA), nicotinamide riboside (NR), and nicotinamide mononucleotide (NMN) or can be salvaged through the NAD+ salvage pathway, which is crucial for recycling the metabolites of biochemical reactions to replete NAD+ stores in the body [3][20]. An extracellular conversion of NMN to NR by CD73, a cell surface enzyme, represents another mechanism by which the intracellular NAD+ content is maintained [4][21].
Cellular levels of NAD+ are also regulated by the enzyme nicotinamide N-methyltransferase (NNMT). When NNMT methylates nicotinamide, creating methylnicotinamide (MNT), the amount of free nicotinamide is reduced and, therefore, not available for conversion into NAD+ through the NAD+ salvage pathway. NNMT and MNT have been associated with obesity and diabetes mellitus type two [5][22]. However, NNMT also has been shown to stabilize SIRT1, thereby exerting beneficial metabolic effects and protecting against oxidative stress-induced cellular injury [6][7][23,24]. Methylnicotinamide has also been shown to increase lifespan [8][25]. However, many NNMT inhibitors have been developed, and promising data establish a potential use for these in the treatment of pathologic states including, but not limited to, cancer, obesity, metabolic disorders, and alcohol-related fatty liver disease [5][9][10][11][12][22,26,27,28,29]. Taken together, the interaction between NNMT, MNT, and the pathways they help regulate plays a significant role in NAD+ homeostasis and, thus, the complex disease states that undoubtedly influence the aging process.
2. Nicotinamide Adenine DinucleotideD+, Sirtuins and Longevity-Promoting Pathway
Disruption of proper NAD+ and loss of protective sirtuin activity have emerged as the prime targets for NAD+-based interventions [13][30]. Administration of NAD+ precursors, namely NR and NMN, has been shown to alleviate age-related NAD+ pathology, particularly in the context of age-related diseases [14][15][16][13,31,32]. Aging has been associated with a decreased NAD+/NADH ratio in human plasma through the deterioration of NAD+ stores, rather than an increase in NADH [17][12]. Replenishment of NAD+ rescues mitochondrial regulatory function from NAD+ induced pseudohypoxic mitochondrial stress during aging [18][14].
SIRT1, a member of a protein family that is involved in the cellular response to various stressors, has been shown to be implicated in longevity, but experiments and analysis have yielded mixed and context-dependent results. Nonetheless, high-level athletes exhibit higher telomere length and lowered insulin resistance, correlating with higher levels of SIRT1 expression [19][33]. The beneficial activity of SIRT1 may rely on the deacetylation and subsequent activation of the Forkhead transcription factors FoxO and PGC1α [20][21][34,35]. FoxOs is a transcription factor involved in stress resistance, cell cycle arrest, apoptosis, and tumor suppression. Activation of FoxOs has been linked with longevity in worms and flies [22][23][36,37]. Important processes, including growth, development, metabolism, reproduction, and longevity, are regulated through insulin/insulin-like growth factor signaling (IIS) working through the activity of FoxO. This pathway has been shown to extend neuronal activity and longevity under low IIS conditions [24][25][38,39]. PGC1α exerts its influence on mitochondrial biogenesis, where deficits are apparent in metabolic disease states. Overexpression of PGC1α has shown improved insulin sensitivity in muscle [26][27][28][40,41,42]. AMPK, involved in energy expenditure, also exhibits similarity to and bi-directional innervation from SIRT1 and further inhibits mTOR, an inhibitory process that has also been linked to longevity. It further activates SIRT1 by increasing available NAD+ stores [29][43]. Furthermore, nuclear factor κB (NF-κB) signaling, a process involved in innate immunity, can be inhibited by SIRT1 activity to reduce prolonged inflammatory signaling [30][44]. Depending on NAD+ stores in the body, SIRT1 activity arises as an interesting target in age-related pathway manipulation to promote longevity (Figure 1) [15][31][32][33][31,45,46,47]. The importance of maintaining an adequate NAD+ level for optimal SIRT1 activity during aging may be a key factor in regulating longevity.
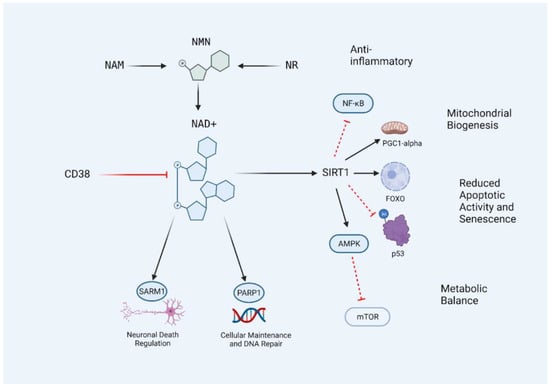
Figure 1. The CD38/NAD+/SIRT1 Axis. NAD+ levels in the body can be influenced by the supplementation of precursors nicotinamide (NAM), nicotinamide riboside (NR), and nicotinamide mononucleotide (NMN). NAD+ levels decrease with age and are further metabolized by the activation of SIRT1, PARP1, SARM1, and CD38. Restoring NAD+ levels allows for an increase in SIRT1 activity due to increased substrate availability, resulting in the inhibition of age-promoting pathways and activation of adaptive and protective transcription factors and processes. The central lineage may be described as the CD38/NAD+/SIRT1 axis, and targeting this access with nutraceutical interventions may prevent the age-related decline of NAD+ levels in the body. Black lines indicate conversion or activation. Red lines indicate inhibitors or destroyers of the indicated target.
3. Nicotinamide Adenine DinucleotideD+ and Circadian Rhythm
NAD+ stores in the body are extremely crucial for the programming of the circadian metabolic clock. Older mice with less abundant NAD+ experienced prolonged repression of CLOCK/BMAL1 transcription compared to younger mice with more abundant NAD+, resulting in disrupted and dampened mitochondrial and transcriptional oscillation [34][48]. NAD+ supplementation and restoration in circadian mutant mice have also been shown to re-establish proper respiratory oscillations and circadian metabolic regulation, particularly through SIRT3 regulatory activity [35][49]. An abundant reserve of NAD+ and proper sirtuin activation are crucial for maintaining the integrity of the various endogenous clocks, and supplementation with NAD+ precursors may potentially alleviate any age-related perturbations in these circadian processes [36][50]. NAD+ deficiencies are observed in these numerous age-related diseases, and NAD+-based interventions are currently underway in an attempt to ameliorate this common conundrum these diseases share [37][38][39][40][51,52,53,54].
4. CD38 and the Decline of Nicotinamide Adenine DinucleotideD+
Adequate intracellular levels of NAD+ are crucial for the proper function of several biological processes, including mitochondria metabolism [41][55]. In mitochondrial disease models, PARP inhibition or NR supplementation ameliorated deficits in metabolism and exercise capacity [42][56]. Strikingly, a cell surface glycoprotein, cluster of differentiation 38 (CD38), also acts as one of the main consumers of NAD+ stores in the body and is involved in immune activation and inflammatory signaling [43][57]. The NAD+ degrading enzyme CD38 has also been shown to regulate SIRT1 activity along with NAD+ availability [44][58]. CD38 expression increases during aging and CD38 knockout models exhibit heightened NAD+ levels and protection against obesity, metabolic disorders, and cancer progression [45][59], illuminating CD38′s role in increasing the risk of developing age-related metabolic diseases [46][60]. It has been suggested that CD38 deficiency may result in susceptibility to autoimmunity and decreased immune integrity, but further studies in humans are required to analyze the effects of insufficient CD38 [47][61]. Nevertheless, reducing chronic inflammation is an aim of healthy aging; inflammatory profiles of senescent cells are associated with heightened CD38 activation and resulting NAD+ degradation through compounding aging and inflammation processes [48][62]. Interestingly, this decline in NAD+ and increase in CD38 with age can potentially be attributed to another age-related phenomenon: the accumulation of senescent cells, cells that exhibit permanent growth arrest. Inflammation and aging, or “inflammaging,” describes the build-up of inflammatory signaling compounds due to cellular senescence and the expression of CD38 as a senescent cellular inflammatory profile. Senescent cells, through their senescence-associated secretory profiles (SASP) of a milieu of IL-6, TNF-α, and CXCL 1 and 2, induce CD38 expression in M1 macrophages, causing age-related NAD+ decline in non-senescent phenotypic populations. These age-related senescent cells have also been shown to accumulate in hepatic and visceral white adipose tissue, and the resulting SASP induces pro-inflammatory M1 macrophage proliferation and enhanced CD38 expression [49][50][51][63,64,65]. The significant interplay between SASP, CD38, and NAD+ levels reveals an interventional target to further enhance available NAD+ in the body, particularly through strong inhibition of CD38 activity or expression well into the aging process to prevent any age-related biological deficits.
5. Nicotinamide MononucleotideN as an Nicotinamide Adenine DinucleotideAD+ Boosting Therapeutic
When using an interventional method to target declining NAD+ levels, the bioavailability and proper uptake of the proposed supplement must be analyzed. NAD+ precursors, such as NR, NMN, and NAM, have been implicated in the adequate uptake and subsequent biosynthesis of NAD+ [52][53][66,67]. For instance, NAD3®, an NR-based dietary supplement, was shown in a clinical trial in older adults to significantly improve NAD+ levels as well as improve blood lipid profiles by decreasing LDL:HDL cholesterol ratios [54][68]. Another meta-analysis revealed that NAD+ boosting mechanisms are successful in decreasing total cholesterol, triglyceride, and the LDL:HDL ratio [55][69]. Moreover, NMN in particular serves as a molecule of interest in the context of NAD+ support, mainly due to the fact that only one conversion step is required to reach NAD+, compared to more required steps for other precursors. NMN supplementation has had numerous benefits, ranging from cardiovascular to neurodegenerative disease contexts, and proper uptake and resulting biosynthesis of NAD+ is observed in varying tissue types [56][57][58][70,71,72]. A successful increase in intracellular NAD+/NADH ratio was evident in a clinical trial in adults aged 40 to 65 years over 60 days at a dosage of 300 mg daily, providing key safety and efficacy data to support the use of NMN UtheverTM as a valid interventional approach to increase NAD+ in a safe manner [59][73]. NMN supplementation has also been shown to target one of the hallmarks of aging, telomere attrition. Short term supplementation of NMN in pre-aging mice and humans, ages 45 to 60 years, showed a significant increase in telomere length in peripheral blood mononuclear cells, as well as altered fecal microbiota benefiting immune and metabolic pathways [60][74], where specific administration in mice has been shown to increase the ratio of beneficial to harmful gut bacteria strains [61][75]. NMN supplementation has also been shown to enhance the miRNA vascular expression profile in aged mice [62][76]. Respiratory parameters and aerobic capacity can also be enhanced through six weeks of NMN supplementation in adult runners, likely attributed to the enhanced oxygen utilization occurring in skeletal muscle as a result of increased NAD+ availability [63][77]. Furthermore, daily NMN supplementation at 250 mg was shown to be well tolerated, efficacious when increasing the NAD+/NADH ratio, and improved muscle function in aged but otherwise healthy men [64][78]. NMN supplementation has also been implicated in improving circadian rhythm patterns in older adults, where 250 mg daily for 12 weeks led to improvements in sleep quality, as measured through PSQI, fatigue, and physical performance [65][79].
Nevertheless, caution is warranted, as more recent data showed that in response to NR administration, increased rates of brain metastasis were observed after intracardial injection of triple-negative breast cancer (TNBC) cells in 9 out of 11 NR-treated mice compared to 3 out of 12 mice treated controls, proposing the increased risk of cancer metastasis in response to NR administration [66][80]. A contrasting study, however, showed no adverse effects on rats after administration of NMN at doses of 375, 750, and 150 mg/kg/d, with a maximum acute dose identified at 2666 mg/kg/d, enhancing the safety profile of NMN supplementation [67][81] To understand if there are differences in the safety of NAD+ precursor use, additional research is needed. To help to understand the translatability and mechanistic actions described in animal models, several clinical trials are ongoing (Table 1).
Table 1. Various clinical trials (completed and ongoing) detailing the use of NMN and other NMN derivates to improve health, metabolic markers, and disease parameters.
| Clinical Trials | Compound of Interest |
|---|---|
| Nicotinamide Mononucleotide Increases Muscle Insulin Sensitivity in Prediabetic Women [68][82] | NMN |
| Effect of Oral Administration of Nicotinamide Mononucleotide on Clinical Parameters and Nicotinamide Metabolite Levels in Healthy Japanese Men [69][83] | NMN |
| Nicotinamide Mononucleotide Supplementation Enhances Aerobic Capacity in Amateur Runners: a randomized, double-blind study [63][77] | NMN |
| Effect of 12-Week Intake of Nicotinamide Mononucleotide on Sleep Quality, Fatigue, and Physical Performance in Older Japanese Adults: a randomized, double-blind placebo-controlled study [65][79] | NMN |
| Safety Evaluation of Beta-nicotinamide Mononucleotide Oral Administration in Healthy Adult Men and Women [70][84] | NMN |
| The Efficacy and Safety of Beta-nicotinamide Mononucleotide (NMN) Supplementation in Healthy Middle-aged Adults: a randomized, multicenter, double-blind, placebo-controlled, parallel-group dose-dependent clinical trial [71][85] | NMN |
| A Multicenter, Randomized, Double-Blind, Parallel Design, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Uthever (NMN Supplement), an Orally Administered Supplementation, in Middle-Aged and Older Adults [59][73] | NMN |
| MIB-626, an Oral Formulation of a Microcrystalline Unique Polymorph of Beta-Nicotinamide Mononucleotide, Increases Circulating Nicotinamide Adenine Dinucleotide and its Metabolome in Middle-Aged and Older Adults [72][86] | MIB-626 |
| Phase 2a MIB-626 vs. Placebo COVID-19 (NCT05038488) | MIB-626 |
| Effect of Oral NAD+ Precursors Administration on Blood NAD+ Concentration in Healthy Adults (NICO) (NCT05517122) | NAM, NR, and NMN |
| Effect of NMN Supplementation on Organ System Biology (VAN) (NCT04571008) | NMN |
| Pharmacodynamics and Tolerance of Nicotinamide Mononucleotide (NMN, 400mg/Day) in Healthy Adults (NCT04862338) | NMN |
| Study to Evaluate the Effect of Nicotinamide Mononucleotide (NMN) As an Adjuvant to Standard of Care (SOC) On Fatigue Associated with Covid-19 Infection (NCT05175768) | NMN |
| Nicotinamide Mononucleotide in Hypertensive Patients (NCT04903210) | NMN |
| Safety and Pharmacokinetics of Nicotinamide Mononucleotide (NMN) in Healthy Adults (NCT04910061) | NMN |
| Effect of NMN (Nicotinamide Mononucleotide) on Polycystic Ovary Syndrome (NMN) (NCT05305677) | NMN |
| Effect of NMN (Nicotinamide Mononucleotide) on Diminished Ovarian Reserve (Including Premature Ovarian Insufficiency) (NCT05485610) | NMN |
| Effect of NMN on Muscle Recovery and Physical Capacity in Healthy Volunteers with Moderate Physical Activity (NCT04664361) | NMN |
6. A Combination Approach for Restoring Youthful Nicotinamide Adenine DinucleotideD+ Levels during Aging
As the interest in geroprotective trends grows, the momentum of clinical trials utilizing NAD+ boosting molecules is increasing. However, proper metrics evaluating the benefits and efficacy of intervention of NAD+ boosting supplements must be further standardized to offer significant and informative comparative effects of geroprotective interventions. NAD+ precursors have shown promising effects in human trials (Table 1), but with the involvement of CD38 and SIRT1 pathways, many potential interactions exist to further amplify the NAD+ boosting effects of orthomolecular interventions. SIRT1 activation, NAD+ synergy, CD38 inhibition, and methylation support are all important factors when considering maximizing the potential of NAD+ precursor administration. The synergistic effects and health benefits of the compounds involved in these three processes will be further discussed to create a foundation to pave the way for potentiating supplement formulations for maximal NAD+ boosting effects. One approach to maximize NAD+ boosting capacity would be to supplement safe levels of NMN with other geroprotectors and nutraceuticals that will further enhance endogenous NAD+ levels, ensuring the restoration of physiological levels of NAD+. Therefore, rwesearchers summarized below different geroprotectors that enhance endogenous NAD+ levels but also have their own longevity benefits. For a selection of the following compounds, synergistic health effects of NMN and a geroprotector have been reported, suggesting the benefit of this combinatorial approach.
