In materials (polymer) science and medicinal chemistry, heteroaromatic derivatives play the role of the central skeleton in development of novel devices and discovery of new drugs. On the other hand, (3-trifluoromethyl)phenyldiazirine (TPD) is a crucial chemical method for understanding biological processes such as ligand–receptor, nucleic acid–protein, lipid–protein, and protein–protein interactions. In particular, use of TPD has increased in materials science to create novel electric and polymer devices with comparative ease and reduced costs. Therefore, a combination of heteroaromatics and (3-trifluoromethyl)diazirine is a promising option for creating better materials and elucidating the unknown mechanisms of action of bioactive heteroaromatic compounds.
- diazirine
- heteroaromatics
- medicinal chemistry
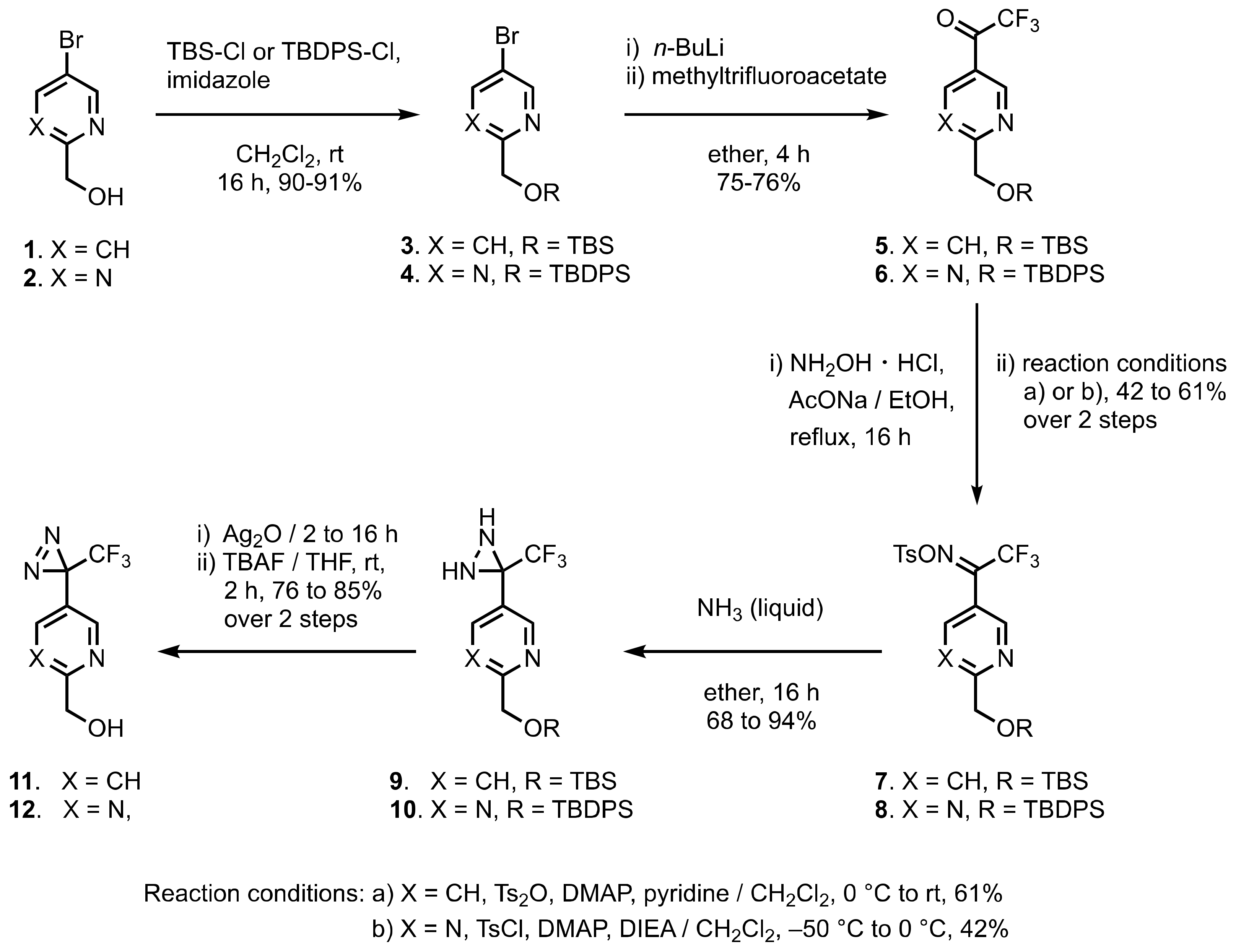
1. Diazirinyl-Substituted Pyridines and Pyrimidines
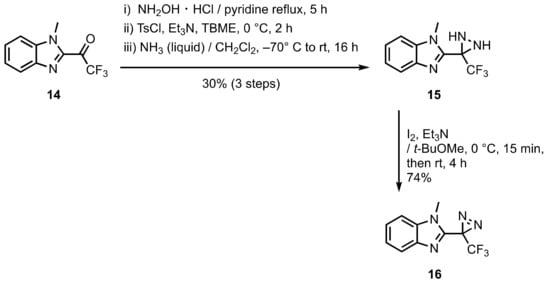
2. Diazirinyl-Substituted Benzimidazoles
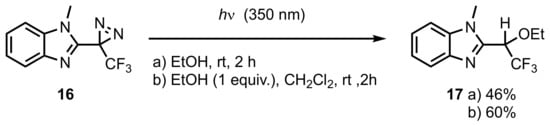
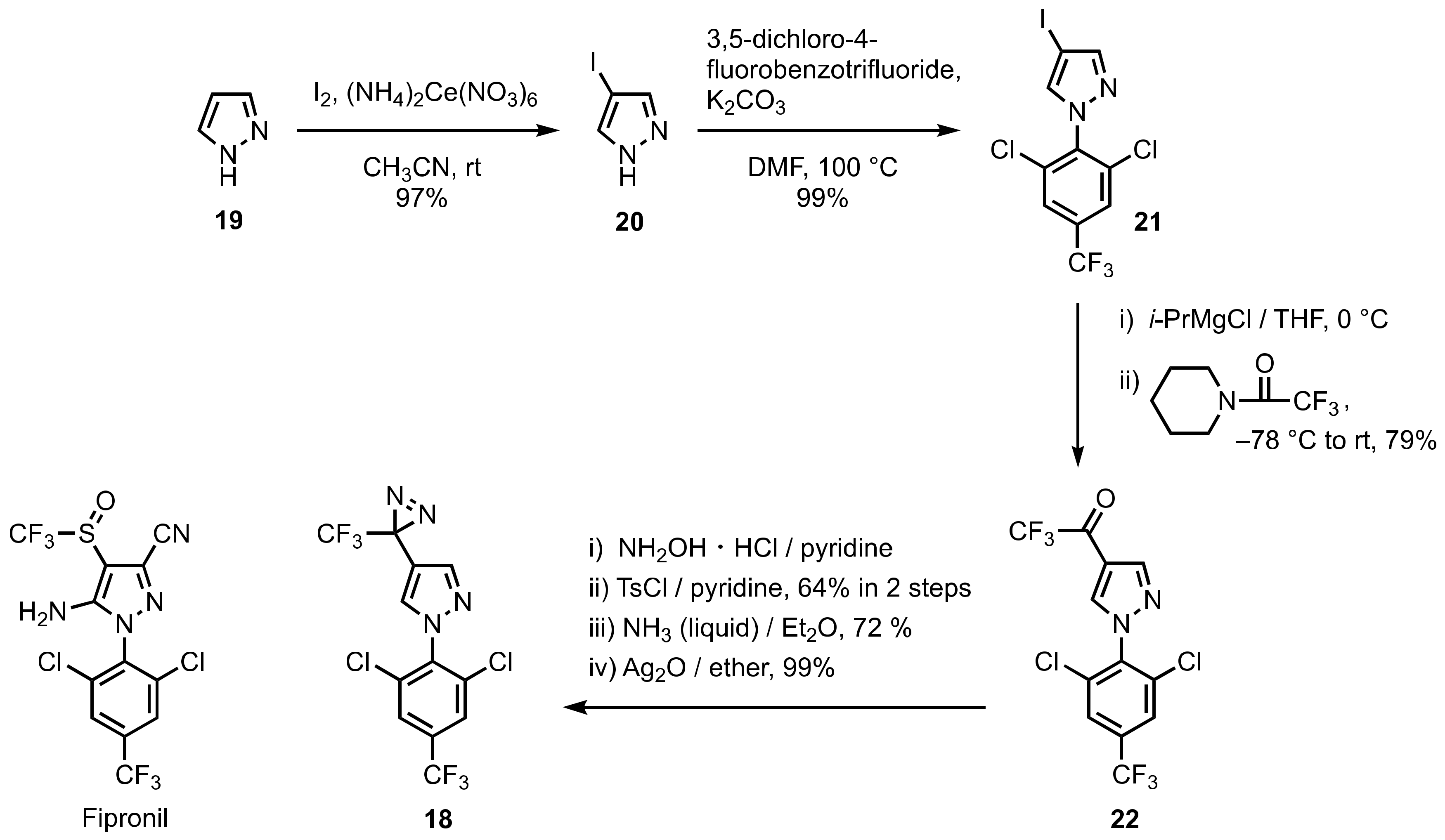
3. Diazirinyl-Substituted Pyrazoles
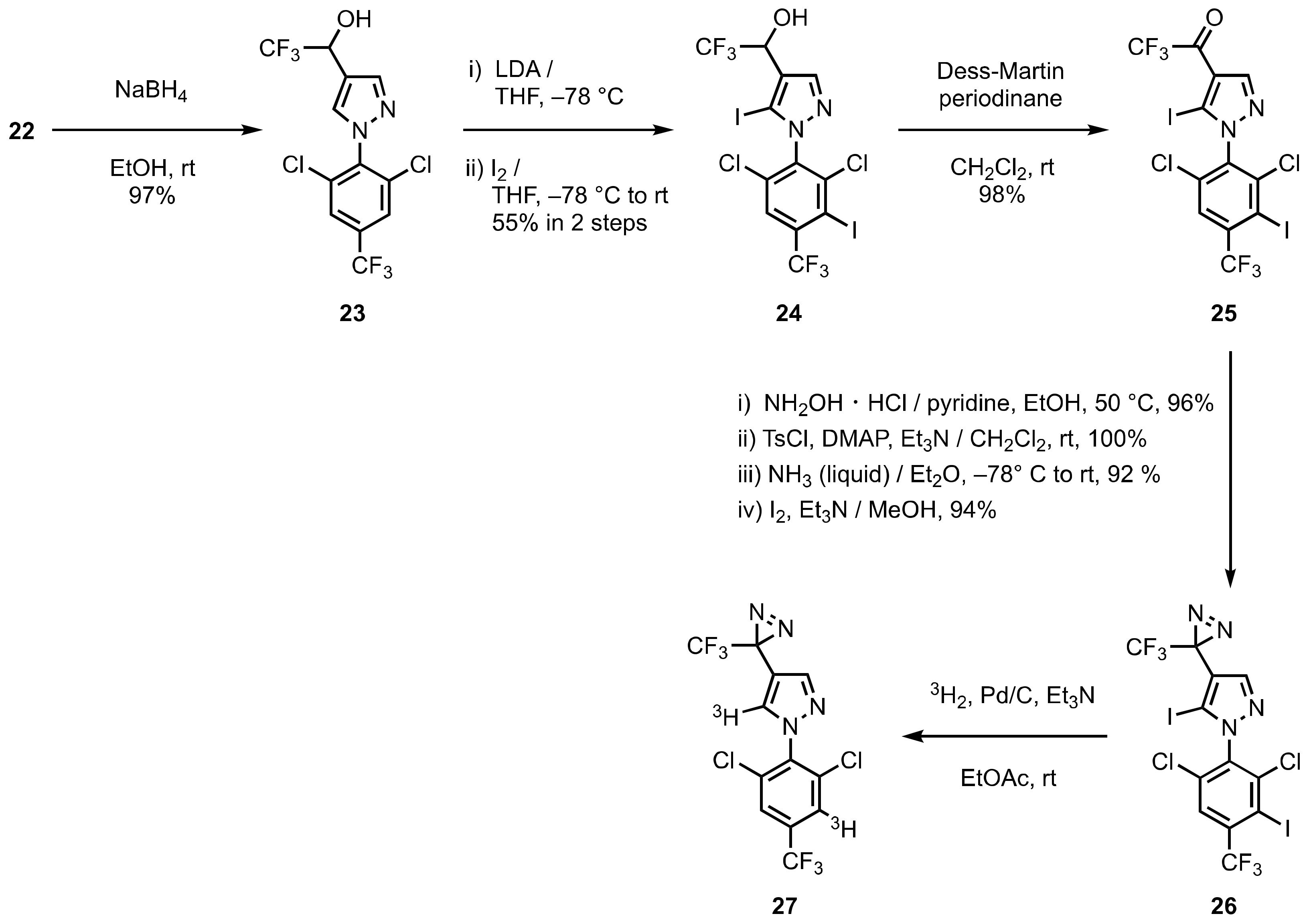
4. Diazirinyl-Substituted Benzoxazolinone
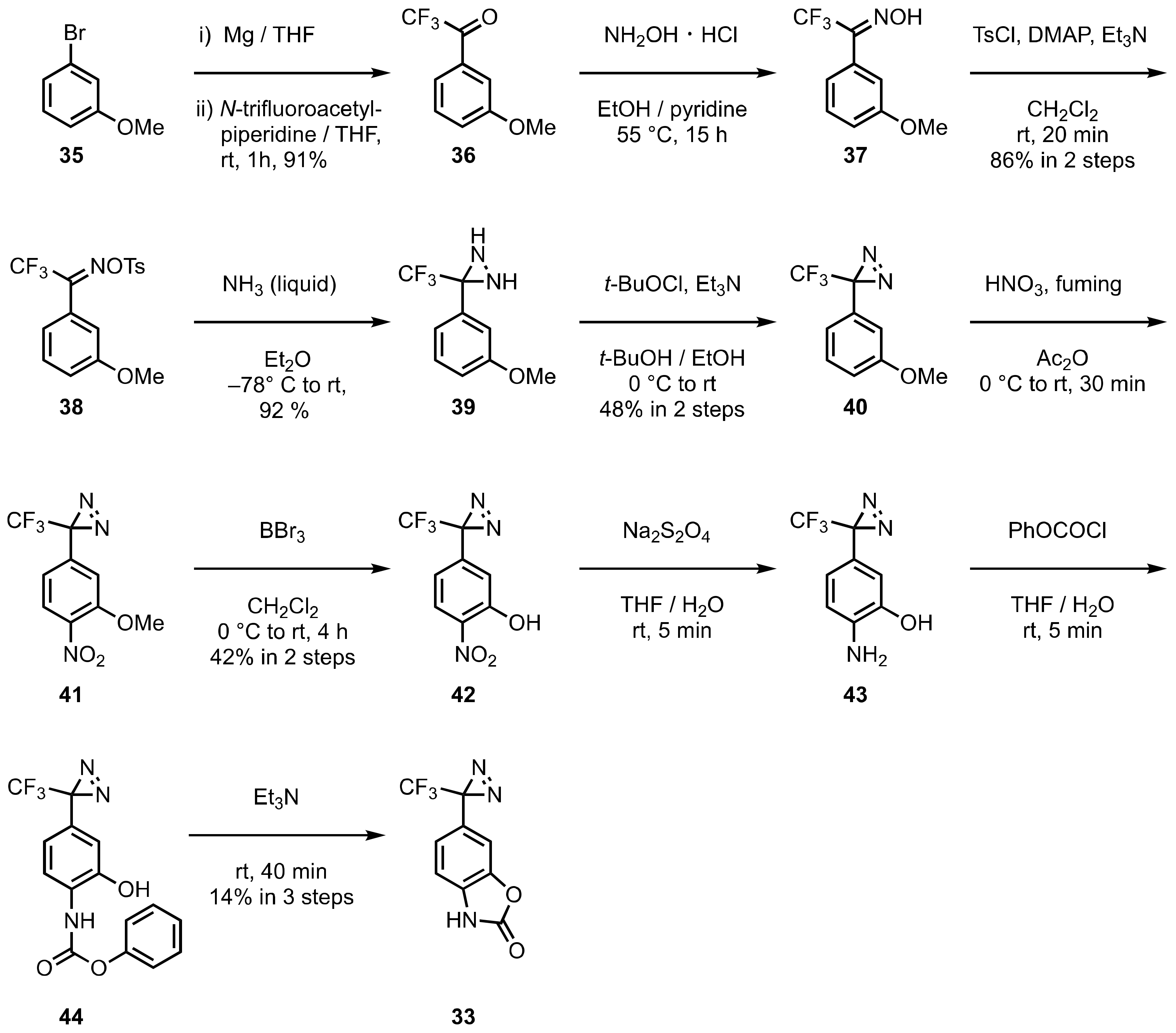
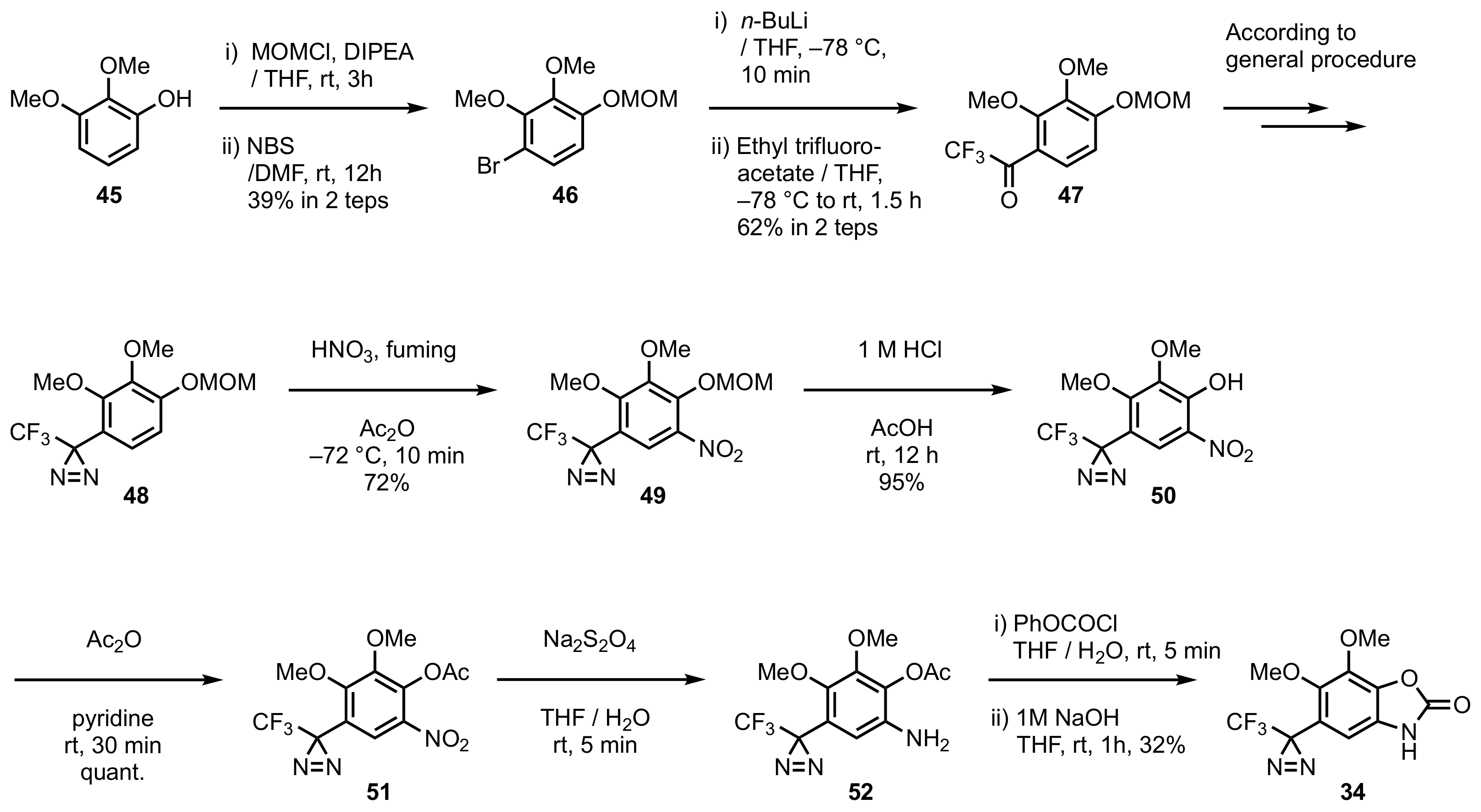
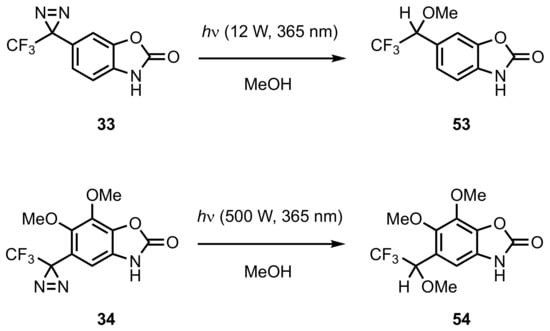
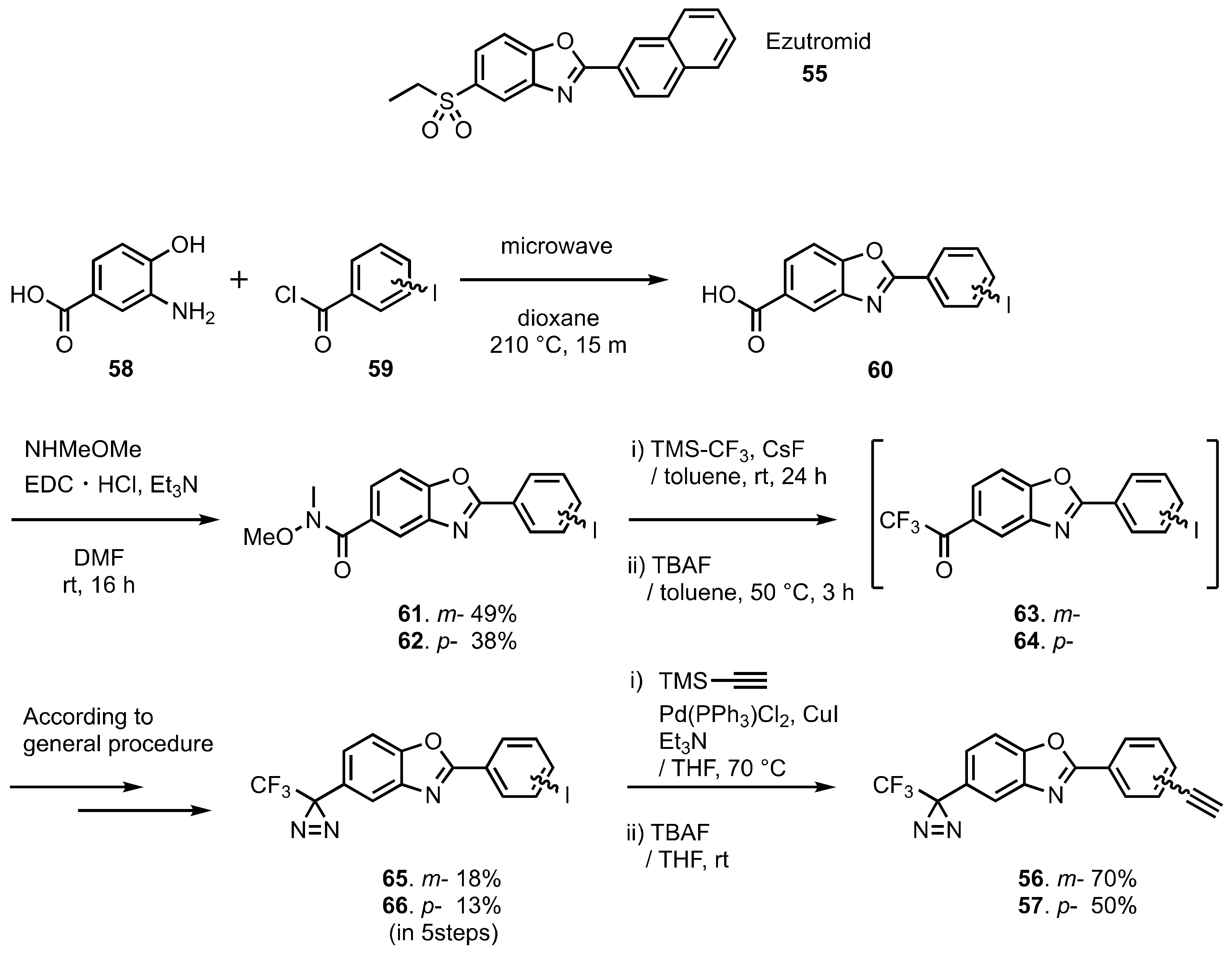
5. Diazirinyl-Substituted Benzoxazole
References
- Dey, K.; Roy Chowdhury, S.; Dykstra, E.; Koronatov, A.; Lu, H.P.; Shinar, R.; Shinar, J.; Anzenbacher, P., Jr. Diazirine-Based Photo-Crosslinkers for Defect Free Fabrication of Solution Processed Organic Light-Emitting Diodes. J. Mater. Chem. C 2020, 8, 11988–11996.
- Chowdhury, S.; Jana, A.; Mandal, A.K.; Choudhary, R.J.; Phase, D.M. Time Evolution of the Structural, Electronic, and Magnetic Phases in Relaxed SrCoO3 Thin Films. ACS Appl. Electron. Mater. 2021, 3, 3060–3071.
- Nazir, R.; Bi, L.; Musolino, S.F.; Margoto, O.H.; Çelebi, K.; Mobuchon, C.; Takaffoli, M.; Milani, A.S.; Falck, G.; Wulf, J.E. Polyamine−Diazirine Conjugates for Use as Primers in UHMWPE−Epoxy Composite Materials. ACS Appl. Polym. Mater. 2022, 4, 1728–1742.
- Yan, Z.; Xu, D.; Lin, Z.; Wang, P.; Cao, B.; Ren, H.; Song, F.; Wan, C.; Wang, L.; Zhou, J.; et al. Highly Stretchable van der Waals Thin Films for Adaptable and Breathable Electronic Membranes. Science 2022, 375, 852–859.
- Kumar, A.B.; Tipton, J.D.; Manetsch, R. 3-Trifluoromethyl-3-Aryldiazirine Photolabels with Enhanced Ambient Light Stability. Chem. Commun. 2016, 52, 2729–2732.
- Cross, R.M.; Monastyrskyi, A.; Mutka, T.S.; Burrows, J.N.; Kyle, D.E.; Manetsch, R. Endochin Optimization: Structure-Activity and Structure-Property Relationship Studies of 3-Substituted 2-Methyl-4(1H)-quinolones with Antimalarial Activity. J. Med. Chem. 2010, 53, 7076–7094.
- Wang, Z.; Deng, X.; Xiong, S.; Xiong, R.; Liu, J.; Zou, L.; Lei, X.; Cao, X.; Xie, Z.; Chen, Y.; et al. Design, Synthesis and Biological Evaluation of Chrysin Benzimidazole Derivatives as Potential Anticancer Agents. Nat. Prod. Res. 2018, 32, 2900–2909.
- Morais, G.R.; Palma, E.; Marques, F.; Gano, L.; Oliveira, M.C.; Abrunhosa, A.; Miranda, H.V.; Outeiro, T.F.; Santos, I.; Paulo, A. Synthesis and Biological Evaluation of Novel 2-Aryl Benzimidazoles as Chemotherapeutic Agents. J. Heterocycl. Chem. 2017, 54, 255–267.
- Shaker, Y.M.; Omar, M.A.; Mahmoud, K.; Elhallouty, S.M.; El-Senousy, W.M.; Ali, M.M.; Mahmoud, A.E.; Abdel-Halim, A.H.; Soliman, S.M.; El Diwani, H.I. Synthesis, in Vitro and in Vivo Aantitumor and Antiviral Activity of Novel 1-Substituted Benzimidazole Derivatives. J. Enzyme Inhib. Med. Chem. 2015, 30, 826–845.
- Onnis, V.; Demurtas, M.; Deplano, A.; Balboni, G.; Baldisserotto, A.; Manfredini, S.; Pacifico, S.; Liekens, S.; Balzarini, J. Design, Synthesis and Evaluation of Antiproliferative Activity of New Benzimidazolehydrazones. Molecules 2016, 21, 579.
- Çevik, U.A.; Sağlık, B.N.; Korkut, B.; Özkay, Y.; Ilgınc, S. Antiproliferative, Cytotoxic, and Apoptotic Effects of New Benzimidazole Derivatives Bearing Hydrazone Moiety. J. Heterocycl. Chem. 2018, 55, 138–148.
- Abd el Al, S.N.; Soliman, F.M.A. Synthesis, Some Reactions, Cytotoxic Evaluation and Antioxidant Study of Novel Benzimidazole Derivatives. Der Pharma Chem. 2015, 7, 71–84.
- Bellam, M.; Gundluru, M.; Sarva, S.; Chadive, S.; Netala, V.R.; Tartte, V.; Cirandur, S.R. Synthesis and Antioxidant Activity of Some New N-Alkylated Pyrazole Containing Benzimidazoles. Chem. Heterocycl. Compd. 2017, 53, 173–178.
- Sharma, R.; Bali, A.; Chaudhari, B.B. Synthesis of Methanesulphonamido-Benzimidazole Derivatives as Gastro-Sparing Antiinflammatory Agents with Antioxidant Effect. Bioorg. Med. Chem. Lett. 2017, 27, 3007–3013.
- Sethi, P.; Bansal, Y.; Bansal, G. Synthesis and PASS-Assisted Evaluation of Coumarin–Benzimidazole Derivatives as Potential Anti-Inflammatory and Anthelmintic Agents. Med. Chem. Res. 2018, 27, 61–71.
- Yang, H.; Ren, Y.; Gao, X.; Gao, Y. Synthesis and Anticoagulant Bioactivity Evaluation of 1,2,5-Trisubstituted Benzimidazole Fluorinated Derivatives. Chem. Res. Chin. Univ. 2016, 32, 973–978.
- Wang, F.; Ren, Y.-J. Design, Synthesis, Biological Evaluation and Molecular Docking of Novel Substituted 1-Ethyl-1H-Benzimidazole Fluorinated Derivatives as Thrombin Inhibitors. J. Iran. Chem. Soc. 2016, 13, 1155–1166.
- Vandeputte, M.M.; Van Uytfanghe, K.; Layle, N.K.; St Germaine, D.M.; Iula, D.M.; Stove, C.P. Synthesis, Chemical Characterization, and μ-Opioid Receptor Activity Assessment of the Emerging Group of “Nitazene” 2-Benzylbenzimidazole Synthetic Opioids. ACS Chem. Neurosci. 2021, 12, 1241–1251.
- Raimer, B.; Wartmann, T.; Jones, P.G.; Lindel, T. Synthesis, Stability, and Photoreactivity of Diazirinyl-Substituted N-Heterocycles Based on Indole, Benzimidazole, and Imidazole. Eur. J. Org. Chem. 2014, 2014, 5509–5520.
- Cheng, H.; Pei, Y.; Leng, F.; Li, J.; Liang, A.; Zou, D.; Wu, Y.; Wu, Y. Highly Efficient Synthesis of Aryl and Heteroaryl Trifluoromethyl Ketones via o-iodobenzoic acid (IBX). Tetrahedron Lett. 2013, 54, 4483–4486.
- Moss, R.A.; Perez, L.A.; Turro, N.J.; Gould, I.R.; Hacker, N.P. Hammett Analysis of Absolute Carbene Addition Rate Constants. Tetrahedron Lett. 1983, 24, 685–688.
- Mueller, P.H.; Rondan, N.G.; Houk, K.N.; Harrison, J.F.; Hooper, D.; Willen, B.H.; Liebman, J.F. Carbene Singlet-Triplet Gaps. Linear Correlations with Substituent Donation. J. Am. Chem. Soc. 1981, 103, 5049–5052.
- Creary, X. Regioselectivity in the Addition of Singlet and Triplet Carbenes to 1,1-Dimethylallene. A Probe for Carbene Multiplicity. J. Am. Chem. Soc. 1980, 102, 1611–1618.
- Wang, J.; Kubicki, J.; Peng, H.; Platz, M.S. Influence of Solvent on Carbene Intersystem Crossing Rates. J. Am. Chem. Soc. 2008, 130, 6604–6609.
- Faisal, M.; Saeed, A.; Hussain, S.; Dar, P.; Larik, F.A. Recent Developments in Synthetic Chemistry and Biological Activities of Pyrazole Derivatives. J. Chem. Sci. 2019, 131, 70.
- Costa, R.F.; Turones, L.C.; Cavalcante, K.V.N.; Rosa Júnior, I.A.; Xavier, C.H.; Rosseto, L.P.; Napolitano, H.B.; Castro, P.F.D.S.; Neto, M.L.F.; Galvão, G.M.; et al. Heterocyclic Compounds: Pharmacology of Pyrazole Analogs from Rational Structural Considerations. Front. Pharmacol. 2021, 12, 666725.
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124.
- Faisal, M.; Hussain, S.; Haider, A.; Saeed, A.; Laril, F.A. Assessing the Effectiveness of Oxidative Approaches for the Synthesis of Aldehydes and Ketones from Oxidation of Iodomethyl Group. Chem. Pap. 2019, 73, 1053–1067.
- Ran, F.; Liu, Y.; Zhang, D.; Liu, M.; Zhao, G. Discovery of Novel Pyrazole Derivatives as Potential Anticancer Agents in MCL. Bioorg. Med. Chem. Lett. 2019, 29, 1060–1064.
- Sony Jacob, K.; Swastika, G. A Battle Against Aids: New Pyrazole Key to an Older Lock-reverse Transcriptase. Int. J. Pharm. Pharm. Sci. 2016, 8, 75–79.
- Kumar, S.; Gupta, S.; Rani, V.; Sharma, P. Pyrazole Containing Anti-HIV Agents: An Update. Med. Chem. 2022, 18, 831–846.
- Bekhit, A.A.; Hassan, A.M.; Abd El Razik, H.A.; El-Miligy, M.M.; El-Agroudy, E.J.; Bekhit, A.-D. New Heterocyclic Hybrids of Pyrazole and Its Bioisosteres: Design, Synthesis and Biological Evaluation as Dual Acting Antimalarial-Antileishmanial Agents. Eur. J. Med. Chem. 2015, 94, 30–44.
- Heller, S.T.; Natarajan, S.R. 1,3-Diketones from Acid Chlorides and Ketones: A Rapid and General One-Pot Synthesis of Pyrazoles. Org. Lett. 2006, 8, 2675–2678.
- Chebib, M.; Johnston, G.A. GABA-Activated Ligand Gated Ion Channels: Medicinal Chemistry and Molecular Biology. J. Med. Chem. 2000, 43, 1427–1447.
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M., Jr.; Kim, J.J.; Hibbs, R.E. Structure of a Human Synaptic GABAA Receptor. Nature 2018, 559, 67–72.
- Singh, N.S.; Sharma, R.; Singh, S.K.; Singh, D.K. A Comprehensive Review of Environmental Fate and Degradation of Fipronil and Its Toxic Metabolites. Environ. Res. 2021, 199, 111316.
- Sirisoma, N.S.; Ratra, G.S.; Tomizawa, M.; Casida, J.E. Fipronil-Based Photoaffinity Probe for Drosophila and Human Beta 3 GABA Receptors. Bioorg. Med. Chem. Lett. 2001, 11, 2979–2981.
- Sammelson, R.E.; Casida, J.E. Synthesis of a Tritium-Labeled, Fipronil-Based, Highly Potent, Photoaffinity Probe for the GABA Receptor. J. Org. Chem. 2003, 68, 8075–8079.
- Delfino, J.M.; Schreiber, S.L.; Richards, F.M. Design, Synthesis, and Properties of a Photoactivatable Membrane-Spanning Phospholipidic Probe. J. Am. Chem. Soc. 1993, 115, 3458–3474.
- Weber, T.; Brunner, J. 2-(Tributylstannyl)-4-benzyl Alcohol: A Building Block for Photolabeling and Cross-Linking Reagents of Very High Specific Radioactivity. J. Am. Chem. Soc. 1995, 117, 3084–3095.
- Li, G.; Samadder, P.; Arthur, G.; Bittmana, R. Synthesis and Antiproliferative Properties of a Photoactivatable Analogue of ET-18-OCH3. Tetrahedron 2001, 57, 8925–8932.
- Leroux, F.; Schlosser, M. The “Aryne” Route to Biaryls Featuring Uncommon Substituent Patterns. Angew. Chem. Int. Ed. 2002, 41, 4272–4274.
- Dess, D.B.; Martin, J.C. Readily Accessible 12-I-5 Oxidant for the Conversion of Primary and Secondary Alcohols to Aldehydes and Ketones. J. Org. Chem. 1983, 48, 4155–4156.
- Linderman, R.J.; Graves, D.M. Oxidation of Fluoroalkyl-Substituted Carbinols by the Dess-Martin. J. Org. Chem. 1989, 54, 661–668.
- Kosemura, S.; Emori, H.; Yamamura, S.; Anai, T.; Aizawa, H.; Ohtake, N.; Hasegawa, K. Isolation and Characterization of 4-Chloro-6,7-dimethoxybenzoxazolin-2-one, A New Auxin-inhibiting Benzoxazolinone from Zea mays. Chem. Lett. 1995, 24, 1053–1054.
- Hoshi-Sakoda, M.; Usui, K.; Ishizuka, K.; Kosemura, S.; Yamamura, S.; Hasegawa, K. Structure-Activity Relationships of Benzoxazolinones with Respect to Auxin-Induced Growth and Auxin-Binding Protein. Phytochemistry 1994, 37, 297–300.
- Kosemura, S.; Emori, H.; Yamamura, S.; Anai, T.; Tomita, K.; Hasegawa, K. Design of Photoaflinity Reagents for Labeling the Auxin Receptor in Maize. Tetrahedron Lett. 1997, 38, 2125–2128.
- Hatanaka, Y.; Hashimoto, M.; Nakayama, H.; Kanaoka, Y. Synthesis of Nitro-Substituted Aryl Diazirines. An Entry to Chromogenic Carbene Precursors for Photoaffinity Labeling. Chem. Pharm. Bull. 1994, 42, 826–831.
- Hatanaka, Y.; Hashimoto, M.; Kurihara, H.; Nakayama, H.; Kanaoka, Y. A Novel Family of Aromatic Diazirines for Photoaffinity Labeling. J. Org. Chem. 1994, 59, 383–387.
- Kohler, M.; Clarenbach, C.F.; Bahler, C.; Brack, T.; Russi, E.W.; Bloch, K.E. Disability and Survival in Duchenne Muscular Dystrophy. J. Neurol. Neurosurg. Psychiatry 2009, 80, 320–325.
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Han, J.J.; Escolar, D.M.; Florence, J.M.; Duong, T.; Arrieta, A.; Clemens, P.R.; Hoffman, E.P.; et al. Cinrg Investigators. The Cooperative International Neuromuscular Research Group Duchenne Natural History Study—A Longitudinal Investigation in the Era of Glucocorticoid Therapy: Design of Protocol and the Methods Used. Muscle Nerve 2013, 48, 32–54.
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 1: Diagnosis, and Pharmacological and Psychosocial Management. Lancet Neurol. 2010, 9, 77–93.
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 2: Implementation of Multidisciplinary Care. Lancet Neurol. 2010, 9, 177–189.
- Moxley, R.T., III; Pandya, S.; Ciafaloni, E.; Fox, D.J.; Campbell, K. Change in Natural History of Duchenne Muscular Dystrophy with Long-Term Corticosteroid Treatment: Implications for Management. J. Child Neurol. 2010, 25, 1116–1129.
- Guiraud, S.; Roblin, D.; Kay, D.E. The Potential of Utrophin Modulators for the Treatment of Duchenne Muscular Dystrophy. Expert Opin. Orphan. Drugs 2018, 6, 179–192.
- Wilkinson, I.V.L.; Perkins, K.J.; Dugdale, H.; Moir, L.; Vuorinen, A.; Chatzopoulou, M.; Squire, S.E.; Monecke, S.; Lomow, A.; Geese, M.; et al. Chemical Proteomics and Phenotypic Profiling Identifies the Aryl Hydrocarbon Receptor as a Molecular Target of the Utrophin Modulator Ezutromid. Angew. Chem. Int. Ed. 2020, 59, 2420–2428.
- Medvedev, A.; Kopylov, A.; Buneeva, O.; Zgoda, V.; Archakov, A. Affinity-Based Proteomic Profiling: Problems and Achievements. Proteomics 2012, 12, 621–637.
- Shi, H.; Zhang, C.J.; Chen, G.Y.; Yao, S.Q. Cell-Based Proteome Profiling of Potential Dasatinib Targets by Use of Affinity-Based Probes. J. Am. Chem. Soc. 2012, 134, 3001–3014.
- Won, S.J.; Eschweiler, J.D.; Majmudar, J.D.; Chong, F.S.; Hwang, S.Y.; Ruotolo, B.T.; Martin, B.R. Affinity-Based Selectivity Profiling of an In-Class Selective Competitive Inhibitor of Acyl Protein Thioesterase 2. ACS Med. Chem. Lett. 2016, 8, 215–220.
- Chancellor, D.R.; Davies, K.E.; De Moor, O.; Dorgan, C.R.; Johnson, P.D.; Lambert, A.G.; Lawrence, D.; Lecci, C.; Maillol, C.; Middleton, P.J.; et al. Discovery of 2-Arylbenzoxazoles as Upregulators of Utrophin Production for the Treatment of Duchenne Muscular Dystrophy. J. Med. Chem. 2011, 54, 3241–3250.
- Rudzinski, D.M.; Kelly, C.B.; Leadbeater, N.E. A Weinreb Amide Approach to the Synthesis of Trifluoromethylketones. Chem. Commun. 2012, 48, 9610–9612.
- Hill, J.R.; Robertson, A.A.B. Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis. J. Med. Chem. 2018, 61, 6945–6963.
