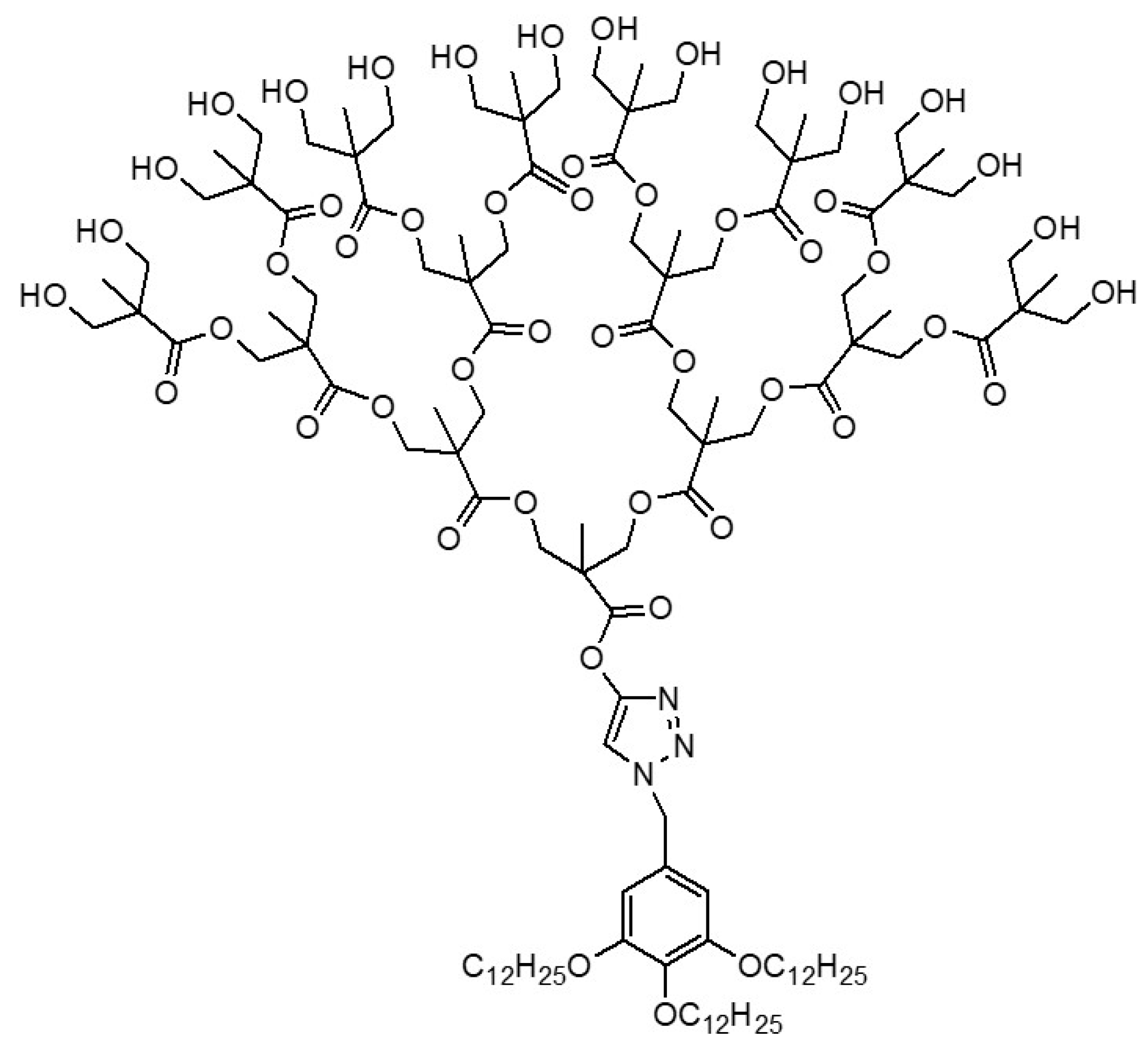
Amphiphilic Janus dendrimers (JDs) can be defined as dendritic macromolecules made of two dendrons with opposite polarities, which either differ from each other by their terminal groups, or their structures differ entirely. Amphiphilic JDs are named following a more or less general scheme that encompass in this order, the lipophilic dendrons, the core if exists, and the hydrophilic dendrons. The unique architectures of amphiphilic Janus dendrimers, with multifunctional terminal groups, different structures of branches in a single molecule (beside the capacity of self-assembly in aqueous media forming dendrimersomes, which in turn benefit from properties like predictable size and thickness, stability and biocompatibility), constitute premises for a wide range of biomedical applications where conventional dendrimers have failed.
- Janus dendrimers
- dendrimersomes
- glycodendrimersomes
- stabilizing agents
- nanocarriers
- gene delivery
1. Janus Dendrimers as Stabilizing Agents
1.1. Drug Suspensions
1.2. Au and Ag Nanoparticles
2. Janus Dendrimers as Biological Membrane Mimics
2.1. Amphiphilic Janus Dendrimers (JDs)
2.2. Janus glycodendrimers (JGDs)
3. Janus Dendrimers as Nanocarriers
4. Janus Dendrimers as Protein Recruitment Enhancers
5. Vectors for Gene Delivery
5.1. DNA
5.2. Messenger RNA
5.3. Small Interference RNAs
References
- Selin, M.; Nummelin, S.; Deleu, J.; Ropponen, J.; Viitala, T.; Lahtinen, M.; Koivisto, J.; Hirvonen, J.; Peltonen, L.; Kostiainen, M.A.; et al. High-Generation Amphiphilic Janus-Dendrimers as Stabilizing Agents for Drug Suspensions. Biomacromolecules 2018, 19, 3983–3993.
- Zhao, L.; Ling, Q.; Liu, X.; Hang, C.; Zhao, Q.; Liu, F.; Gu, H. Multifunctional triazolylferrocenyl Janus dendron: Nanoparticle stabilizer, smart drug carrier and supramolecular nanoreactor. Appl. Organometal. Chem. 2018, 32, e4000.
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665.
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653.
- Ciganda, R.; Gu, H.; Hernandez, R.; Escobar, A.; Martínez, A.; Yates, L.; Moya, S.; Ruiz, J.; Astruc, D. Electrostatic Assembly of Functional and Macromolecular Ferricinium Chloride-Stabilized Gold Nanoparticles. Inorg. Chem. 2017, 56, 2784–2791.
- Rapakousiou, A.; Deraedt, C.; Gu, H.; Salmon, L.; Belin, C.; Ruiz, J.; Astruc, D. Mixed-Valent Click Intertwined Polymer Units Containing Biferrocenium Chloride Side Chains Form Nanosnakes that Encapsulate Gold Nanoparticles. J. Am. Chem. Soc. 2014, 136, 13995–13998.
- Deraedt, C.; Rapakousiou, A.; Wang, Y.; Salmon, L.; Bousquet, M.; Astruc, D. Multifunctional Redox Polymers: Electrochrome, Polyelectrolyte, Sensor, Electrode Modifier, Nanoparticle Stabilizer, and Catalyst Template. Angew. Chem. Int. Ed. 2014, 53, 8445–8449.
- Li, N.; Zhao, P.; Igartua, M.E.; Rapakousiou, A.; Salmon, L.; Moya, S.; Ruiz, J.; Astruc, D. Stabilization of AuNPs by monofunctional triazole linked to ferrocene, ferricenium, or coumarin and applications to synthesis, sensing, and catalysis. Inorg. Chem. 2014, 53, 11802–11808.
- Wang, Y.; Salmon, L.; Ruiz, J.; Astruc, D. Metallodendrimers in three oxidation states with electronically interacting metals and stabilization of size-selected gold nanoparticles. Nat. Commun. 2014, 5, 3489.
- Ciganda, R.; Irigoyen, J.; Gregurec, D.; Hernández, R.; Moya, S.; Wang, C.; Ruiz, J.; Astruc, D. Liquid–Liquid Interfacial Electron Transfer from Ferrocene to Gold(III): An Ultrasimple and Ultrafast Gold Nanoparticle Synthesis in Water under Ambient Conditions. Inorg. Chem. 2016, 55, 6361–6363.
- Gu, H.; Ciganda, R.; Castel, P.; Vax, A.; Gregurec, D.; Irigoyen, J.; Moya, S.; Salmon, L.; Zhao, P.; Ruiz, J.; et al. Redox-Robust Pentamethylferrocene Polymers and Supramolecular Polymers, and Controlled Self-Assembly of Pentamethylferricenium Polymer-Embedded Ag, AgI, and Au Nanoparticles. Chem. Eur. J. 2015, 21, 18177–18186.
- Rapakousiou, A.; Deraedt, C.; Irigoyen, J.; Wang, Y.; Pinaud, N.; Salmon, L.; Ruiz, J.; Moya, S.; Astruc, D. Synthesis and Redox Activity of “Clicked” Triazolylbiferrocenyl Polymers, Network Encapsulation of Gold and Silver Nanoparticles and Anion Sensing. Inorg. Chem. 2015, 54, 2284–2299.
- Wang, H.; Wang, X.; Winnik, M.A.; Manners, I.J. Redox-Mediated Synthesis and Encapsulation of Inorganic Nanoparticles in Shell-Cross-Linked Cylindrical Polyferrocenylsilane Block Copolymer Micelles. J. Am. Chem. Soc. 2008, 130, 12921–12930.
- Jia, L.; Tong, L.; Liang, Y.; Petretic, A.; Guerin, G.; Manners, I.; Winnik, M.A. Templated Fabrication of Fiber-Basket Polymersomes via Crystallization-Driven Block Copolymer Self-Assembly. J. Am. Chem. Soc. 2014, 136, 16676–16682.
- Sui, X.; Feng, X.; Di Luca, A.; van Blitterswijk, C.A.; Moroni, L.; Hempenius, M.A.; Vancso, G.J. Poly(N-isopropylacrylamide)–poly(ferrocenylsilane) dual-responsive hydrogels: Synthesis, characterization and antimicrobial applications. Polym. Chem. 2013, 4, 337–342.
- Sui, X.; Shui, L.; Cui, J.; Xie, Y.; Song, J.; van den Berg, A.; Hempenius, M.A.; Vancso, G.J. Redox-responsive organometallic microgel particles prepared from poly(ferrocenylsilane)s generated using microfluidics. Chem. Commun. 2014, 50, 3058–3060.
- Liu, Y.; Mu, S.; Liu, X.; Ling, Q.; Hang, C.; Ruiz, J.; Astruc, D.; Gu, H. Ferrocenyl Janus mixed-dendron stars and their stabilization of Au and Ag nanoparticles. Tetrahedron 2018, 74, 4777–4789.
- Enciso, A.E.; Doni, G.; Nifosì, R.; Palazzesi, F.; Gonzalez, R.; Ellsworth, A.A.; Coffer, J.L.; Walker, A.V.; Pavan, G.M.; Mohamed, A.A.; et al. Facile synthesis of stable, water soluble, dendron-coated gold nanoparticles. Nanoscale 2017, 9, 3128.
- Shi, P.; Qu, Y.; Liu, C.; Khan, H.; Sun, P.; Zhang, W. Redox-Responsive Multicompartment Vesicles of Ferrocene-Containing Triblock Terpolymer Exhibiting On–Off Switchable Pores. ACS Macro Lett. 2016, 5, 88–93.
- Morsbach, J.; Elbert, J.; Rüttiger, C.; Winzen, S.; Frey, H.; Gallei, M. Polyvinylferrocene-Based Amphiphilic Block Copolymers Featuring Functional Junction Points for Cross-Linked Micelles. Macromolecules 2016, 49, 3406–3414.
- Sherman, S.E.; Xiao, Q.; Percec, V. Mimicking complex biological membranes and their programmable glycan ligands with dendrimersomes and glycodendrimersomes. Chem. Rev. 2017, 117, 6538–6631.
- Torre, P.; Xiao, Q.; Buzzacchera, I.; Sherman, S.E.; Rahimi, K.; Kostina, N.Y.; Rodriguez-Emmenegger, C.; Möller, M.; Wilson, C.J.; Klein, M.L.; et al. Encapsulation of hydrophobic components in dendrimersomes and decoration of their surface with proteins and nucleic acids. Proc. Natl. Acad. Sci. USA 2019, 116, 15378–15385.
- Yadavalli, S.S.; Xiao, Q.; Sherman, S.E.; Hasley, W.D.; Klein, M.L.; Goulian, M.; Percec, V. Bioactive cell-like hybrids from dendrimersomes with a human cell membrane and its components. Proc. Natl. Acad. Sci. USA 2019, 116, 744–752.
- Xiao, Q.; Ludwig, A.-K.; Romanò, C.; Buzzacchera, I.; Sherman, S.E.; Vetro, M.; Vértesy, S.; Kaltner, H.; Reed, E.H.; Möller, M.; et al. Exploring functional pairing between surface glycoconjugates and human galectins using programmable glycodendrimersomes. Proc. Natl. Acad. Sci. USA 2018, 115, E2509–E2518.
- Rodriguez-Emmenegger, C.; Xiao, Q.; Kostina, N.Y.; Sherman, S.E.; Rahimi, K.; Partridge, B.E.; Li, S.; Sahoo, D.; Reveron Perez, A.M.; Buzzacchera, I.; et al. Encoding biological recognition in a bicomponent cell-membrane mimic. Proc. Natl. Acad. Sci. USA 2019, 116, 5376–5382.
- Murphy, P.V.; Romero, A.; Xiao, Q.; Ludwig, A.-K.; Jogula, S.; Shilova, N.V.; Singh, T.; Gabba, A.; Javed, B.; Zhang, D.; et al. Probing sulfatide-tissue lectin recognition with functionalized glycodendrimersomes. Iscience 2021, 24, 101919.
- Peterca, M.; Percec, V.; Leowanawat, P.; Bertin, A. Predicting the Size and Properties of Dendrimersomes from the Lamellar Structure of Their Amphiphilic Janus Dendrimers. J. Am. Chem. Soc. 2011, 133, 20507–20520.
- Zhang, S.; Moussodia, R.O.; Sun, H.J.; Leowanawat, P.; Muncan, A.; Nusbaum, C.D.; Chelling, K.M.; Heiney, P.A.; Klein, M.L.; André, S.; et al. Mimicking biological membranes with programmable glycan ligands self-assembled from amphiphilic Janus glycodendrimers. Angew. Chem. Int. Ed. 2014, 53, 10899–10903.
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952.
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric lipid hybrid nanoparticles (plns) as emerging drug delivery platform—A comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics 2021, 13, 1291.
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloids Interface Sci. Commun. 2022, 46, 100570.
- Thoma, J.; Belegrinou, S.; Rossbach, P.; Grzelakowski, M.; Kita-Tokarczyk, K.; Meier, W. Membrane protein distribution in composite polymer-lipid thin films. Chem. Commun. 2012, 48, 8811–8813.
- Xia, T.; Hao, W.; Shang, Y.; Xu, S.; Liu, H. Incorporation of Amphipathic Diblock Copolymer in Lipid Bilayer for Improving pH Responsiveness. Int. J. Polym. Sci. 2016, 2016, 5879428.
- Buzzacchera, I.; Xiao, Q.; Han, H.; Rahimi, K.; Li, S.; Kostina, N.Y.; Toebes, B.J.; Wilner, S.E.; Möller, M.; Rodriguez-Emmenegger, C.; et al. Screening libraries of amphiphilic Janus dendrimers based on natural phenolic acids to discover monodisperse Unilamellar Dendrimersomes. Biomacromolecules 2019, 20, 712–727.
- Joseph, A.; Wagner, A.M.; Garay-Sarmiento, M.; Aleksanyan, M.; Haraszti, T.; Söder, D.; Georgiev, V.N.; Dimova, R.; Percec, V.; Rodriguez-Emmenegge, C. Zwitterionic Dendrimersomes: A Closer Xenobiotic Mimic of Cell Membranes. Adv. Mater. 2022, 34, 2206288.
- Kostina, N.Y.; Rahimi, K.; Xiao, Q.; Haraszti, T.; Dedisch, S.; Spatz, J.P.; Schwaneberg, U.; Klein, M.L.; Percec, V.; Möller, M.; et al. Membrane-mimetic Dendrimersomes engulf living bacteria via endocytosis. Nano Lett. 2019, 19, 5732–5738.
- Kostina, N.Y.; Wagner, A.M.; Haraszti, T.; Rahimi, K.; Xiao, Q.; Klein, M.L.; Percec, V.; Rodriguez-Emmenegger, C. Unraveling topology-induced shape transformations in dendrimersomes. Soft Matter 2021, 17, 254–267.
- Bi, F.; Zhang, J.; Wei, Z.; Yu, D.; Zheng, S.; Wang, J.; Li, H.; Hua, Z.; Zhang, H.; Yang, G. Dynamic Glycopeptide Dendrimers: Synthesis and Their Controllable Self-Assembly into Varied Glyco-Nanostructures for the Biomimicry of Glycans. Biomacromolecules 2022, 23, 128–139.
- Xiao, Q.; Delbianco, M.; Sherman, S.E.; Perez, A.M.R.; Bharate, P.; Pardo-Vargas, A.; Rodriguez-Emmenegger, C.; Kostina, N.Y.; Rahimi, K.; Söder, D.; et al. Nanovesicles displaying functional linear and branched oligomannose self-assembled from sequence-defined Janus glycodendrimers. Proc. Natl. Acad. Sci. USA 2020, 117, 11931–11939.
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427.
- Krishnan, N.; Perumal, D.; Atchimnaidu, S.; Harikrishnan, K.S.; Golla, M.; Kumar, N.M.; Kalathil, J.; Krishna, J.; Vijayan, D.K.; Varghese, R. Galactose-Grafted 2D Nanosheets from the Self-Assembly of Amphiphilic Janus Dendrimers for the Capture and Agglutination of Escherichia coli. Chem. Eur. J. 2020, 26, 1037–1041.
- Kostina, N.Y.; Söder, D.; Haraszti, T.; Xiao, Q.; Rahimi, K.; Partridge, B.E.; Klein, M.L.; Percec, V.; Rodriguez-Emmenegger, C. Enhanced concanavalin A binding to preorganized mannose nanoarrays in glycodendrimersomes revealed multivalent interactions. Angew. Chem. Int. Ed. 2021, 60, 8352–8360.
- Taabache, S.; Bertin, A. Vesicles from Amphiphilic Dumbbells and Janus Dendrimers: Bioinspired Self-Assembled Structures for Biomedical Applications. Polymers 2017, 9, 280.
- Caminade, A.-M.; Laurent, R.; Delavaux-Nicot, B.; Majoral, J.-P. “Janus” dendrimers: Syntheses and properties. New J. Chem. 2012, 36, 217–226.
- Sikwal, D.R.; Kalhapure, R.S.; Govender, T. An emerging class of amphiphilic dendrimers for pharmaceutical and biomedical applications: Janus amphiphilic dendrimers. Eur. J. Pharm. Sci. 2017, 97, 113–134.
- Najafi, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Synthesis of amphiphilic Janus dendrimer and its application in improvement of hydrophobic drugs solubility in aqueous media. Eur. Polym. J. 2020, 134, 109804.
- Lancelot, A.; Clavería-Gimeno, R.; Velázquez-Campoy, A.; Abian, O.; Serrano, J.L.; Sierra, T. Nanostructures based on ammonium-terminated amphiphilic Janus dendrimers as camptothecin carriers with antiviral activity. Eur. Polym. J. 2017, 90, 136–149.
- Percec, V.; Wilson, D.A.; Leowanawat, P.; Wilson, C.J.; Hughes, A.D.; Kaucher, M.S.; Hammer, D.A.; Levine, D.H.; Kim, A.J.; Bates, F.S.; et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science 2010, 328, 1009–1014.
- Filippi, M.; Martinelli, J.; Mulas, G.; Ferraretto, M.; Teirlinck, E.; Botta, M.; Tei, L.; Terreno, E. Dendrimersomes: A new vesicular nano-platform for MR-molecular imaging applications. Chem. Commun. 2014, 50, 3453–3456.
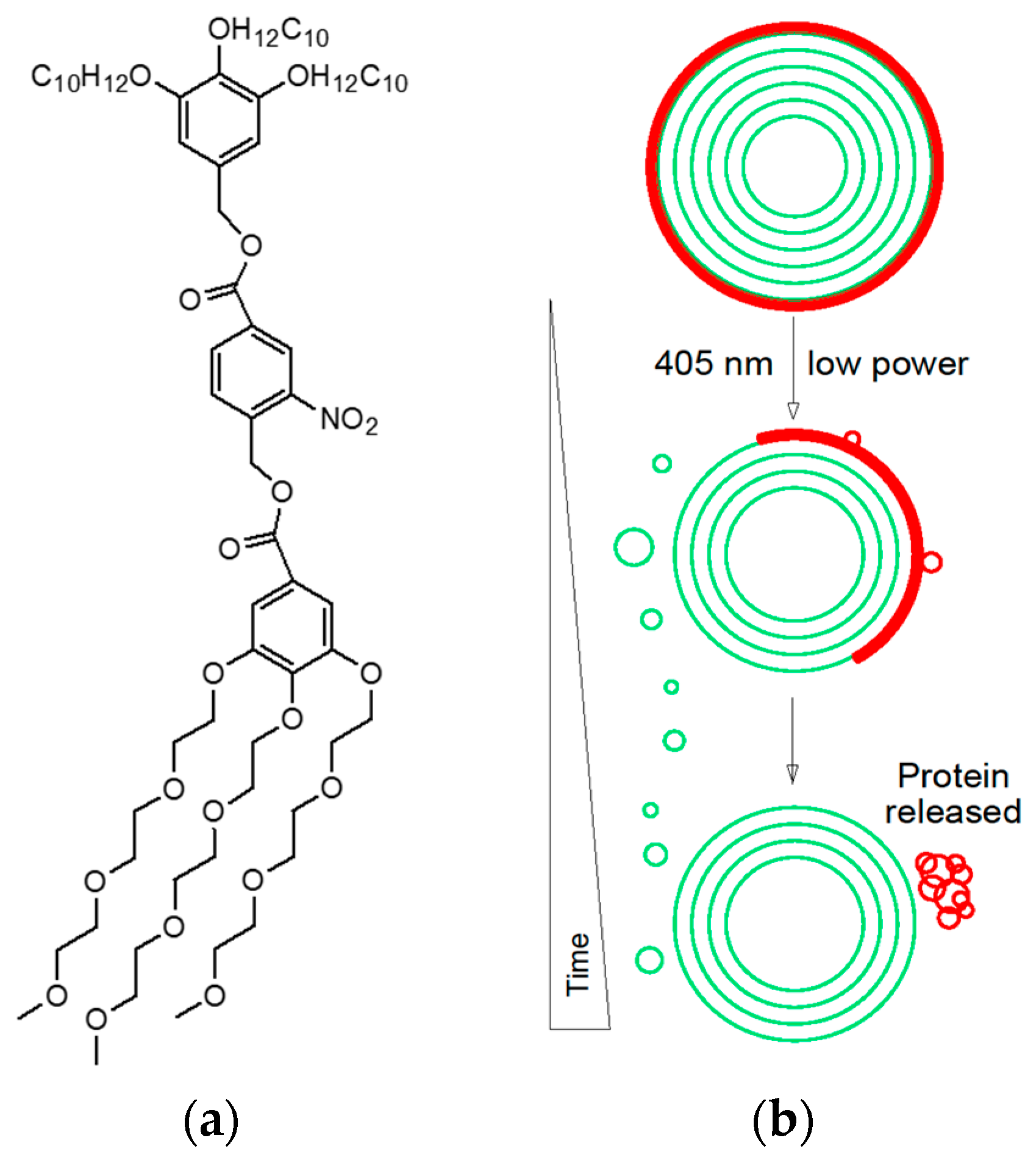
- Nazemi, A.; Gillies, E.R. Dendrimersomes with photodegradable membranes for triggered release of hydrophilic and hydrophobic cargo. Chem. Commun. 2014, 50, 11122–11125.
- Zhang, X.; Rehm, S.; Safont-Sempere, M.M.; Wurthner, F. Vesicular Perylene Dye Nanocapsules as Supramolecular Fluorescent pH Sensor Systems. Nat. Chem. 2009, 1, 623–629.
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983.
- Fedeli, E.; Lancelot, A.; Serrano, J.L.; Calvo, P.; Sierra, T. Self-Assembling Amphiphilic Janus Dendrimers: Mesomorphic Properties and Aggregation in Water. New J. Chem. 2015, 39, 1960–1967.
- Laskar, P.; Somani, S.; Altwaijry, N.; Mullin, M.; Bowering, D.; Warzecha, M.; Keating, P.; Tate, R.J.; Leung, H.Y.; Dufès, C. Redox-sensitive, cholesterol-bearing PEGylated poly(propylene imine)-based dendrimersomes for drug and gene delivery to cancer cells. Nanoscale 2018, 10, 22830–22847.
- Laskar, P.; Somani, S.; Mullin, M.; Tate, R.J.; Warzecha, M.; Bowering, D.; Keating, P.; Irving, C.; Leung, H.Y.; Dufès, C. Octadecyl chain-bearing PEGylated poly(propyleneimine)-based dendrimersomes: Physicochemical studies, redox-responsiveness, DNA condensation, cytotoxicity and gene delivery to cancer cells. Biomater. Sci. 2021, 9, 1431–1448.
- Li, S.; Xia, B.; Javed, B.; Hasley, W.D.; Melendez-Davila, A.; Liu, M.; Kerzner, M.; Agarwal, S.; Xiao, Q.; Torre, P.; et al. Direct visualization of vesicle disassembly and reassembly using photocleavable dendrimers elucidates cargo release mechanisms. ACS Nano 2020, 14, 7398–7411.
- García-Serradilla, M.; Risco, C.; Pacheco, B. Drug repurposing for new, efficient, broad spectrum antivirals. Virus Res. 2020, 264, 22–31.
- San Anselmo, M.; Lancelot, A.; Egido, J.E.; Clavería-Gimeno, R.; Casanova, Á.; Serrano, J.L.; Hernández-Ainsa, S.; Abian, O.; Sierra, T. Janus Dendrimers to Assess the Anti-HCV Activity of Molecules in Cell-Assays. Pharmaceutics 2020, 12, 1062.
- Patil, A.; Mishra, V.; Thakur, S.; Riyaz, B.; Kaur, A.; Khursheed, R.; Patil, K.; Sathe, B. Nanotechnology Derived Nanotools in Biomedical Perspectives: An Update. Curr. Nanosci. 2019, 15, 137–146.
- Su, C.; Liu, Y.; Li, R.; Wu, W.; Fawcett, J.P.; Gu, J. Absorption, distribution, metabolism and excretion of the biomaterials used in Nanocarrier drug delivery systems. Adv. Drug Deliv. Rev. 2019, 143, 97–114.
- Lancelot, A.; González-Pastor, R.; Clavería-Gimeno, R.; Romero, P.; Abian, O.; Martín-Duque, P.; Serrano, J.L.; Sierra, T. Cationic poly(ester amide) dendrimers: Alluring materials for biomedical applications. J. Mater. Chem. B 2018, 6, 3956–3968.
- Castillo-Rodríguez, I.O.; Hernández-Alducin, P.A.; Pedro-Hernández, L.D.; Barajas-Mendoza, I.; Ramírez-Ápan, T.; Martínez-García, M. Antileukemia and Anticolorectal Cancer Activity of Janus Dendrimer Conjugates with Naproxen and Ibuprofen. ChemistrySelect 2023, 8, e20220422.
- Lv, J.; Fan, Q.; Wang, H.; Cheng, Y. Polymers for Cytosolic Protein Delivery. Biomaterials 2019, 218, 119358.
- Lv, S.; Sylvestre, M.; Prossnitz, A.N.; Yang, L.F.; Pun, S.H. Design of Polymeric Carriers for Intracellular Peptide Delivery in Oncology Applications. Chem. Rev. 2021, 121, 11653–11698.
- Cheng, Y. Design of Polymers for intracellular protein and peptide delivery. Chin. J. Chem. 2021, 39, 1443–1449.
- Zhang, Z.; Gao, X.; Li, Y.; Lv, J.; Cheng, Y. Catechol-based polymers with high efficacy in cytosolic protein delivery. CCS Chem. 2022, 1–11.
- Ren, L.; Lv, J.; Wang, H.; Cheng, Y. A Coordinative Dendrimer Achieves Excellent Efficiency in Cytosolic Protein and Peptide Delivery. Angew. Chem. Int. Ed. 2020, 59, 4711–4719.
- Lv, J.; Liu, C.; Lv, K.; Wang, H.; Cheng, Y. Boronic acid-rich dendrimer for efficient intracellular peptide delivery. Sci. China Mater. 2020, 63, 620–628.
- Ren, L.; Gao, Y.; Cheng, Y. A manganese (II)-based coordinative dendrimer with robust efficiency in intracellular peptide delivery. Bioact. Mater. 2022, 9, 44–53.
- Rong, G.; Wang, C.; Hu, J.; Li, Y.; Cheng, Y. Benzaldehyde-tethered fluorous tags for cytosolic delivery of bioactive peptides. J. Control. Release 2022, 351, 703–712.
- Choi, S.-J.; Kwon, S.H.; Lim, Y.-B. 3D2 Self-Assembling Janus Peptide Dendrimers with Tailorable Supermultivalency. Adv. Funct. Mater. 2019, 29, 1808020.
- Falanga, A.; Del Genio, V.; Kaufman, E.A.; Zannella, C.; Franci, G.; Weck, M.; Galdiero, S. Engineering of Janus-Like Dendrimers with Peptides Derived from Glycoproteins of Herpes Simplex Virus Type 1: Toward a Versatile and Novel Antiviral Platform. Int. J. Mol. Sci. 2021, 22, 6488.
- Wang, L.; Shi, C.; Wang, X.; Guo, D.; Duncan, T.M.; Luo, J. Zwitterionic Janus Dendrimer with distinct functional disparity for enhanced protein delivery. Biomaterials 2019, 215, 119233.
- Xiao, Q.; Rivera-Martinez, N.; Raab, C.J.; Bermudez, J.G.; Good, M.C.; Klein, M.L.; Percec, V. Co-assembly of liposomes, Dendrimersomes, and Polymersomes with amphiphilic Janus dendrimers conjugated to Mono- and Tris-Nitrilotriacetic Acid (NTA, TrisNTA) enhances protein recruitment. Giant 2022, 9, 100089.
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8.
- Apartsin, E.; Venyaminova, A.; Majoral, J.-P.; Caminade, A.-M. Dendriplex-Impregnated Hydrogels with Programmed Release Rate. Front. Chem. 2022, 9, 780608.
- Gonçalves, G.A.R.; Paiva, R.M.A. Gene therapy: Advances, challenges and perspectives. Einstein 2017, 15, 369–375.
- Laskar, P.; Somani, S.; Campbell, S.J.; Mullin, M.; Keating, P.; Tate, R.J.; Irving, C.; Leung, H.Y.; Dufès, C. Camptothecin-based dendrimersomes for gene delivery and redoxresponsive drug delivery to cancer cells. Nanoscale 2019, 11, 20058–20071.
- Huang, X.; Kong, N.; Zhang, X.; Cao, Y.; Langer, R.; Tao, W. The landscape of mRNA nanomedicine. Nat. Med. 2022, 28, 2273–2287.
- Chen, J.; Zhu, D.; Liu, X.; Peng, L. Amphiphilic Dendrimer Vectors for RNA Delivery: State-of-the-Art and Future Perspective. Acc. Mater. Res. 2022, 3, 484.
- Zhang, D.; Atochina-Vasserman, E.N.; Maurya, D.S.; Huang, N.; Xiao, Q.; Ona, N.; Liu, M.; Shahnawaz, H.; Ni, H.; Kim, K.; et al. One-Component Multifunctional Sequence-Defined Ionizable Amphiphilic Janus Dendrimer Delivery Systems for mRNA. J. Am. Chem. Soc. 2021, 143, 12315–12327.
- Zhang, D.; Atochina-Vasserman, E.N.; Maurya, D.S.; Liu, M.; Xiao, Q.; Lu, J.; Lauri, G.; Ona, N.; Reagan, E.K.; Ni, H.; et al. Targeted Delivery of mRNA with One-Component Ionizable Amphiphilic Janus Dendrimers. J. Am. Chem. Soc. 2021, 143, 17975–17982.
- Mahmoodi Chalbatani, G.; Dana, H.; Gharagouzloo, E.; Grijalvo, S.; Eritja, R.; Logsdon, C.D.; Memari, F.; Miri, S.R.; Rad, M.R.; Marmari, V. Small interfering RNAs (siRNAs) in cancer therapy: A nano-based approach. Int. J. Nanomed. 2019, 2019, 3111–3128.
- Yonezawa, S.; Koide, H.; Asai, T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles Adv. Drug Deliv. Rev. 2020, 154–155, 64–78.
- Krishnamurthy, S.; Vaiyapuri, R.; Zhang, L.; Chan, J.M. Lipid-coated polymeric nanoparticles for cancer drug delivery. Biomater. Sci. 2015, 3, 923–936.
- Yang, X.-Z.; Dou, S.; Wang, Y.-C.; Long, H.-Y.; Xiong, M.-H.; Mao, C.-Q.; Yao, Y.-D.; Wang, J. Single-step assembly of cationic lipid-polymer hybrid nanoparticles for systemic delivery of siRNA. ACS Nano 2012, 6, 4955–4965.
- Du, X.-J.; Wang, Z.-Y.; Wang, Y.-C. Redox-sensitive dendrimersomes assembled from amphiphilic Janus dendrimers for siRNA delivery. Biomater. Sci. 2018, 6, 2122–2129.
- Chen, C.; Posocco, P.; Liu, X.; Cheng, Q.; Laurini, E.; Zhou, J.; Liu, C.; Wang, Y.; Tang, J.; Col, V.; et al. Mastering dendrimer self-assembly for efficient siRNA delivery: From conceptual design to in vivo efficient gene silencing. Small 2016, 12, 3667–3676.
- Dong, Y.; Yu, T.; Ding, L.; Laurini, E.; Huang, Y.; Zhang, M.; Weng, Y.; Lin, S.; Chen, P.; Marson, D.; et al. A Dual Targeting Dendrimer-Mediated siRNA Delivery System for Effective Gene Silencing in Cancer Therapy. J. Am. Chem. Soc. 2018, 140, 16264–16274.
- Yu, T.; Liu, X.; Bolcato-Bellemin, A.; Wang, Y.; Liu, C.; Erbacher, P.; Qu, F.; Rocchi, P.; Behr, J.; Peng, L. An amphiphilic dendrimer for effective delivery of small interfering RNA and gene silencing in vitro and in vivo. Angew. Chem. Int. Ed. 2012, 51, 8478–8484.
- Liu, X.; Zhou, J.; Yu, T.; Chen, C.; Cheng, Q.; Sengupta, K.; Huang, Y.; Li, H.; Liu, C.; Wang, Y.; et al. Adaptive amphiphilic dendrimer-based nanoassemblies as robust and versatile siRNA delivery systems. Angew. Chem. Int. Ed. 2014, 53, 11822–11827.
- Liu, X.; Wang, Y.; Chen, C.; Tintaru, A.; Cao, Y.; Liu, J.; Ziarelli, F.; Tang, J.; Guo, H.; Rosas, R.; et al. A fluorinated bola-amphiphilic dendrimer for on-demand delivery of siRNA, via specific response to reactive oxygen species. Adv. Funct. Mater. 2016, 26, 8594–8603.