You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by David Garcia-Burgos.
Anorexia nervosa (AN), a serious and often treatment-refractory mental illness characterized by distorted body perception and pathological weight loss due to sustained attempts to restrict food intake, can be understood and addressed through the lens of learning theory; which provides a coherent framework of integrated constructs and principles that describe, explain, and predict how organisms learn and how this learning is translated into behavior.
- modern learning theory
- anorexia nervosa
- associative learning
- conditioning
- eating disorders
- pavlovian conditioning
- Instrumental conditioning
- food-related learning
1. Basic Concepts of Learning Theory
Learning theory is a comprehensive framework that describes, explains, and predicts how organisms learn and translate their learning into behavior. Initially, learning theory was aligned with the behaviorist paradigm (cf. [20,21][1][2]), which only focused on directly observable changes, such as external (motor or psychophysiological) responses. According to behaviorist learning theory, learning occurred through two types of environmental relationships (see [12][3], for an introduction to learning and conditioning), known as classical or Pavlovian conditioning and operant or instrumental conditioning. Pavlovian conditioning involves pairing a neutral stimulus, such as a tone, with an innate unconditioned stimulus, like an electric shock, to produce a conditioned response, such as fear. After the tone has been contingently followed by an electric shock several times, the tone will elicit fear even when no longer followed by the electric shock. At that moment, the tone has become a conditioned stimulus that evokes fear, which is then called the conditioned response. In contrast, operant conditioning involves learning through the relationship between an action and its outcome. An example of instrumental conditioning in a laboratory setting is jumping a barrier to avoid an otherwise imminent electric shock. The action (jumping a barrier) here is instrumental in avoiding the unpleasant outcome (the electric shock). It is important to note that most action–outcome relationships are only valid in the presence of a particular stimulus. This stimulus is called discriminative stimulus and becomes a signal that tells the organism what action is going to become reinforced. For example, a tone becomes a discriminative stimulus when it signals the availability of an electric shock if avoidance is not performed. Ultimately, the distinction between Pavlovian and instrumental conditioning is based on the type of events experienced and the experimental procedure used: conditioned stimulus → unconditioned stimulus versus discriminative stimulus → action → outcome.
Proponents of behaviourist learning theory intentionally ignored what goes on inside “the black box” of the learning organism [22][4]. Other authors opposed this approach, positing that the changes that observe in studies of learning may not directly mirror what the organism has learned. Thus, when the dominance of the behaviourist paradigm declined, internal processes gained recognition [23][5]. Today, modern learning theory explains changes in behaviour by internal processes during Pavlovian and instrumental conditioning in terms of associations between mental representations of stimuli and responses in memory (see Figure 1). Indeed, “conditioning is now described as the learning of relations among events so as to allow the organism to represent its environment” ([24][6], p. 151). With regard to mental representations, this term is used to refer to any model of external or internal events in memory [25][7] and may include information about specific sensory cues (e.g., visual or gustatory properties of a candy), affective values (e.g., pleasant sensations when we eat a candy), motivational properties (e.g., the satiation and nutritive impact) and specific response-eliciting characteristics (e.g., salivation) [26][8].
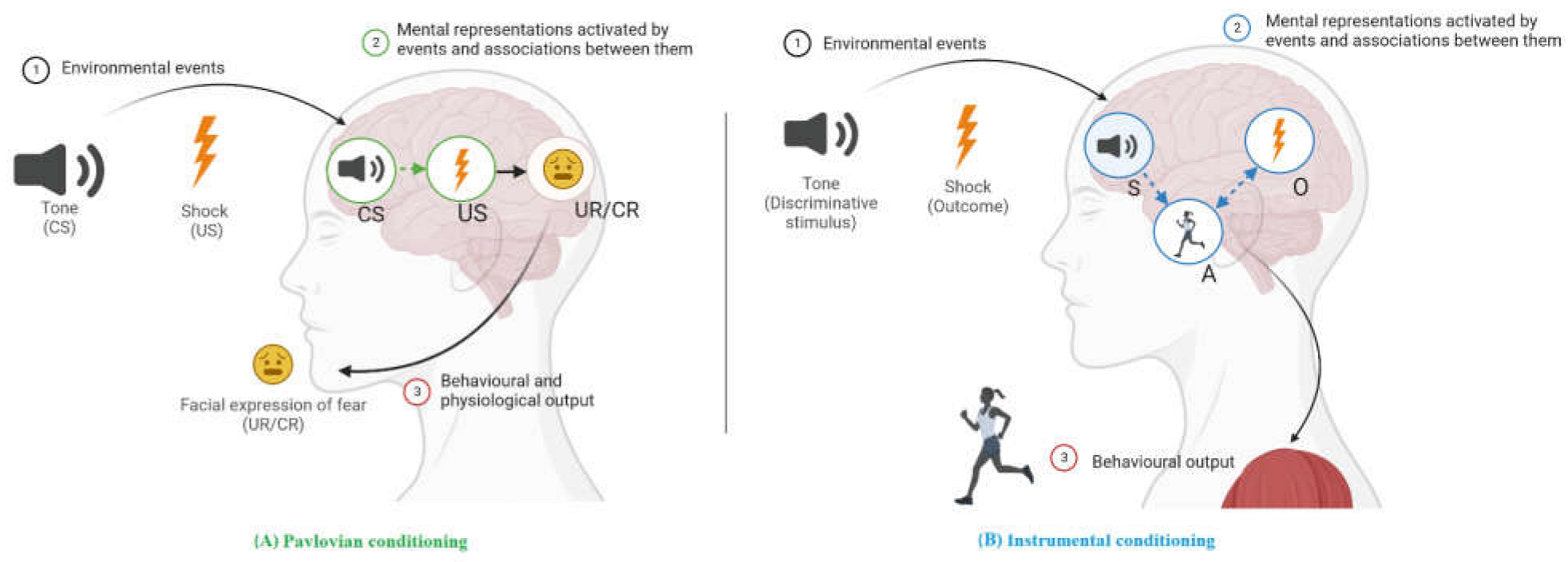
Figure 1. Content of Pavlovian (A) and instrumental (B) learning showing the mental representations and associations acquired after the conditioning experience. (A) Pavlovian conditioning is viewed as involving conditioned stimuli (CS) and unconditioned stimuli (US), such as the pairing of a tone with an electric shock. These pairings result in a CS→US association in memory (in green) through which the tone elicits fear responses such as facial expressions of fear. (B) Instrumental conditioning (in blue) in which a response (e.g., jumping) is followed by an outcome (e.g., an electric shock) and results in an action–outcome (A→O) association. After many repetitions, a new habitual stimulus–action (S→A) association is formed, such as between the tone and jumping. Note: Circles represent mental representations in memory. Lines suggest how one can influence another: solid lines indicate innate links and dashed lines indicate links that can be strengthened or weakened by experience. Activation is shown by an arrow. A: instrumental action; CR: conditioned response; CS: conditioned stimulus; O: outcome; S: discriminative stimulus; UR: unconditioned response; US: unconditioned stimulus. Created in Biorender.com.
With the return of cognition in learning, modern learning theory overcomes the limitations of early learning models of AN, which were overly simplistic and have been justifiably criticised. Thus, the distinction between Pavlovian and instrumental conditioning is not only based on the type of events experienced and the experimental procedure used, but also includes what subjects learn (i.e., mental representations and learned associations in memory). On the other hand, unlike behaviourist positions, modern learning theory does not claim that anything can be learned or that all behaviour is learned, but rather the realisation that our biological systems and associative vulnerabilities constrain what do or do not learn, promoting the learning of specific associations [27][9]. Surprisingly, progress in learning theory has not had a significant impact on clinical research and practice in EDs until very recently.
1.1. Exploring Cognitive Factors in Modern Learning Theory for Anorexia Nervosa
Cognitive factors have a significant impact on learning and performance in intricate ways. In the first case, to the extent that learning is reconceptualized cognitively as mental representations that are created, assembled and/or modified to better reflect the external environment [28][10], anorexia nervosa (AN) may be first characterised by the creation of unhealthy representations. Here, an example is the overvaluation of eating, weight and/or shape, which are considered to be the core psychopathology underlying AN [2][11]. In the case of the assembly of abnormal mental representations, an example may be the food-related phenomenon of thought–shape fusion, specific and distinct cognitive distortions present in patients with eating disorders. It occurs when thinking about eating high-caloric food leads individuals to feel fatter (e.g., “just thinking about eating a chocolate bar can make me gain weight”) [29][12]. An explanation advanced by modern learning theory is that activating the mental representation of sweet–fat foods will excite the feared consequences of eating as well, including the internal body sensations, via a link with the catastrophic weight gain representation. Conversely, given that one of the simplest forms of thought is an association in terms of mental representations of two events [30][13], maladaptive negative thoughts in AN (e.g., ”if I’m fat, people won’t like me”) may be understood as an association between two representations (fatness with social aversive experiences), resulting in exaggerated or pathological responses. Finally, with the introduction of cognition into learning, environment stimuli do not impose the content of learning mechanically on us; rather it opens new opportunities for an active role in the associative process. Thus, for instance, it has been suggested that people can acquire associations by engaging in rule-based processing based on language and formal reasoning [31][14].
In the second case related to performance, cognitive factors also influence responding; for instance, in the control of food-related behaviours [32][15]. Indeed, eating behaviour is often subject to sophisticated cognitive eating controls. One of the most widely practised forms of cognitive control over food intake is dieting, i.e., attempting to restrict intake as a means of weight regulation [33][16]. In AN patients, these cognitive regulations are especially important to overcome hunger sensations after long periods of deprivation. The problem is that anything that disrupts the cognitive control in people with a restricted diet (e.g., BP-AN) appears to unleash overeating [34][17]. Regarding the interplay between the cognitive content of learning and voluntary cognitive control processes in the context of food responses, both can be understood by a sequential pathway through a default-interventionist approach. Simpler automatic associative responses start and then high-level processes are recruited when the simpler responses prove inadequate, particularly when conflict is detected [32][15]. An example of conflict is when BP-AN patients refrain from their automatic tendency to eat attractive and pleasant chocolate in order to maintain incompatible goals in terms of weight status.
2. Progression of the Learning Models for Anorexia Nervosa
In early learning models, AN was seen as particular manifestations of an anxiety disorder. The assumption was that the pathological restriction of eating reduces anxiety (see [35,36][18][19]). This is well illustrated by the conceptualisation of AN as a weight phobia [37,38][20][21] in which patients limit their diet because they are anxious about weight gain. These theoretical models were mainly inspired by the two-factor fear theory (cf. [39][22]) combining Pavlovian and instrumental conditioning. Patients first showed a conditioned fear response through Pavlovian conditioning (Factor 1): caloric food-related cues occurring with weight gain (unconditioned stimulus) act as a warning stimulus (conditioned stimulus) of becoming fat, which elicits the anxiety/fear response (conditioned response). In a second phase, patients begin to diet and restrict their caloric food intake (action) in order to avoid weight gain and the conditioned fear response. Such avoidance via dieting is then negatively reinforced through anxiety reduction (Factor 2). Ironically, despite the significant impact of the two-factor model in learning theory, it has never been directly tested for AN. Indeed, most of the studies in the conditioning basis of AN are descriptive and/or case reports (>65%; can be seen Appendix AApendix in original context). There is only scarce and indirect evidence (see [35][18]). Moreover, within the two-factor model, it was difficult to explain why some patients with AN continuously restricted their calorie intake, even to the point of life-threatening starvation, and why certain patients, who wanted to restrict their calorie intake to lose weight, binge ate on a regular basis. Finally, the two-factor model itself underwent severe criticism (discussed elsewhere, [40,41,42][23][24][25]). As a result, the behaviourist learning perspective fell out of favour as a relevant model for AN. There has recently been renewed interest in the anxiety-based model of conditioned avoidance for adults and adolescents with AN [43,44][26][27]. These new models still posit that avoidance behaviours are acquired responses with the aim of reducing eating-related anxiety. An innovation is that AN is now assumed to develop from vulnerabilities in emotional learning and memory processing. For instance, it has been proposed that patients with AN learn fear more easily than their healthy counterparts [45][28]. In addition, a wider range of conditioning experiences is now taken into account to explain how AN develops and is maintained, such as direct classical conditioning (e.g., food cues and traumatic experiences), verbal conditioning through information (e.g., threatening information about high-calorie food and overweight), vicarious conditioning (e.g., observing others with high-calorie food fears) and/or operant conditioning (e.g., when eating is followed by aversive consequences such as negative judgement from others or criticism) [46][29]. Likewise, other models based on learning processes such as the transdiagnostic theory for the treatment of eating disorders or the reward-centred model for the development and maintenance of AN have been described more recently (as discussed elsewhere; [47][30]). By contrast, the modern associative account of learning provides a much richer picture. For instance, abnormal behaviour is supposed to be activated not only via direct, instructional, verbal or vicarious pathways, but also by novel events that only share physical, perceptual or conceptual features with those representations currently maintained in memory, as well as by indirect, associatively retrieved representations of food stimuli (as observed using mediated learning paradigms; cf. [48][31], for a detailed discussion).References
- Bower, G.H.; Hilgard, E.R. Theories of Learning, 5th ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1981.
- Mowrer, R.R.; Klein, S.B. Handbook of Contemporary Learning Theories; Lawrence Erlbaum: Mahwah, NJ, USA, 2001.
- Schachtman, T.R.; Reilly, S.S. Things you always wanted to know about conditioning but were afraid to ask. In Conditioning and Animal Learning: Human and Non-Human Applications; Schachtman, T., Reilly, S., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 3–23.
- Skinner, B.F. The behavior of organisms; Appleton-Century: New York, NY, USA, 1938.
- Tolman, E.C. Cognitive maps in rats and men. Psychol. Rev. 1948, 55, 189–208.
- Rescorla, R.A. Pavlovian conditioning: It’s not what you think it is. Am. Psychol. 1988, 43, 151–160.
- Arntz, A. A plea for more attention to mental representations. J. Behav. Ther. Exp. Psychiatry 2019, 67, 101510.
- Delamater, A.R.; Oakeshott, S. Learning about Multiple Attributes of Reward in Pavlovian Conditioning. Ann. N. Y. Acad. Sci. 2007, 1104, 1–20.
- Mowrer, R.R.; Klein, S.B. A contrast between traditional and contemporary learning theory. In Contemporary Learning Theories; Klein, S.B., Mowrer, R.R., Eds.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1989; pp. 1–10.
- Howard, R.W. Reconceptualizing learning. Rev. Gen. Psychol. 1999, 3, 251–263.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013.
- Shafran, R.; Teachman, B.A.; Kerry, S.; Rachman, S. A cognitive distortion associated with eating disorders: Thought-shape fusion. Br. J. Clin. Psychol. 1999, 38, 167–179.
- Baumeister, R.F.; von Hippel, W. Meaning and evolution: Why Nature selected human minds to use meaning. Evol. Stud. Imaginative Cult. 2020, 4, 1–18.
- De Houwer, J.; Vandorpe, S.; Beckers, T. On the role of controlled cognitive processes in human associative learning. In New Directions in Human associative Learning; Wills, A.J., Ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2005; pp. 41–63.
- Garcia-Burgos, D. Associative learning and high-level cognitive processes in the control of food-related behaviors. Curr. Opin. Behav. Sci. 2022, 47, 101207.
- Wardle, J. Cognitive control of eating. J. Psychosom. Res. 1988, 32, 607–612.
- Polivy, J.; Herman, C.P. Dieting and binging: A causal analysis. Am. Psychol. 1985, 40, 193.
- Buree, B.U.; Papageorgis, D.; Hare, R.D. Eating in anorexia nervosa and bulimia nervosa: An application of the tripartite model of anxiety. Can. J. Behav. Sci. Rev. Can. Sci. Comport. 1990, 22, 207–218.
- Slade, P. Towards a functional analysis of anorexia nervosa and bulimia nervosa. Br. J. Clin. Psychol. 1982, 21, 167–179.
- Crisp, A.H. Anorexia nervosa, ‘feeding disorder’, ‘nervous malnutrition’, or ‘weight phobia’? World Rev. Nutr. Diet. 1970, 12, 452–504.
- Habermas, T. In defense of weight phobia as the central organizing motive in anorexia nervosa: Historical and cultural arguments for a culture-sensitive psychological conception. Int. J. Eat. Disord. 1996, 19, 317–334.
- Mowrer, O.H.; Lamoreaux, R.R. Fear as an intervening variable in avoidance conditioning. J. Comp. Psychol. 1946, 39, 29–50.
- Cain, C.K. Avoidance problems reconsidered. Curr. Opin. Behav. Sci. 2018, 26, 9–17.
- Gillan, C.M.; Urcelay, G.P.; Robbins, T.W. An associative account of avoidance. In The Wiley Handbook on the Cognitive Neuroscience of Learning; Murphy, R.A., Honey, R.C., Eds.; Wiley Blackwell Chichester: West Sussex, UK, 2016; pp. 442–467.
- LeDoux, J.E.; Moscarello, J.; Sears, R.; Campese, V. The birth, death and resurrection of avoidance: A reconceptualization of a troubled paradigm. Mol. Psychiatry 2016, 22, 24–36.
- Hildebrandt, T.; Bacow, T.; Markella, M.; Loeb, K.L. Anxiety in anorexia nervosa and its management using family-based treatment. Eur. Eat. Disord. Rev. 2010, 20, e1–e16.
- Steinglass, J.E.; Sysko, R.; Glasofer, D.; Albano, A.M.; Simpson, H.B.; Walsh, B.T. Rationale for the application of exposure and response prevention to the treatment of anorexia nervosa. Int. J. Eat. Disord. 2011, 44, 134–141.
- Strober, M. Pathologic fear conditioning and anorexia nervosa: On the search for novel paradigms. Int. J. Eat. Disord. 2004, 35, 504–508.
- Cardi, V.; Leppanen, J.; Mataix-Cols, D.; Campbell, I.C.; Treasure, J. A case series to investigate food-related fear learning and extinction using in vivo food exposure in anorexia nervosa: A clinical application of the inhibitory learning framework. Eur. Eat. Disord. Rev. 2019, 27, 173–181.
- Brytek-Matera, A.; Czepczor, K. Models of eating disorders: A theoretical investigation of abnormal eating patterns and body image disturbance. Arch. Psychiatry Psychother. 2017, 19, 16–26.
- Holland, P.C.; Wheeler, D.S. Representation-mediated food aversions. In Conditioned Taste Aversion: Behavioral and Neural Processes; Reilly, S., Schachtman, T.R., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 196–225.
More
