Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yuhan Liu and Version 2 by Conner Chen.
Nitric oxide (NO) is a gaseous free radical that has been become a potential tool to maintain the quality of postharvest horticultural produce (such as fruits). It plays important roles in delaying ripening, alleviating chilling injury, preventing browning, and enhancing disease resistance. The regulatory function of NO is achieved through the post-transcriptional modification of proteins, such as tyrosine nitration, S-nitrosylation, and nitroalkylation. Secondly, NO can also induce the expression of stress-related genes by synergistically interacting with other signaling substances, such as Ca2+, ethylene (ETH), salicylic acid (SA), and jasmonic acid (JA).
- nitric oxide
- fruits and vegetables
- ripening
1. Introduction
Fruits and vegetables are becoming increasingly important sources of nutrients for humans [1]. However, it is a great challenge to maintain the postharvest quality of fruits and vegetables, including the prevention of pests and diseases, browning after fresh-cutting, softening, and decay, etc. Senescence and environmental stress are the main causes of declines in the postharvest quality of fruits and vegetables [2]. As a natural gas molecule that can freely pass through lipid membranes to regulate plant development and mediate the stress response of plants [3], nitric oxide (NO) has been widely used to control the postharvest quality of fruits and vegetables. Finally, research directions on the effects of NO on postharvest fruits and vegetables meriting future attention are discussed.
The homeostasis of NO in fruits and vegetables is maintained through regulation of its synthesis and degradation (Figure 1). NO can be synthesized via the reductive pathway and oxidative pathway [4][5][4,5]. Nitrite (NO2–) reduction is the major source of NO in plants, which occurs by both enzymatic and non-enzymatic mechanisms [6]. Nitrate reductase (NR) in the cytosol, nitrite–NO reductase (Ni–NOR) in the plasma membrane, nitrate reductase (NiR) in plastids, and xanthine oxidoreductase (XOR) are involved in reductive NO synthesis [7][8][7,8]. Non-enzymatic nitrite reduction occurs spontaneously in the apoplast owing to the acidic conditions or the presence of ascorbic acid or phenols [9][10][9,10]. Several oxidative pathways of NO synthesis have been studied. L-arginine can be oxidized to produce NO by NO synthase-like (NOS-like) enzyme [11], while N-omega hydroxyl-L-arginine is also converted to NO by peroxidase (POD) and hemoglobin. Alternatively, NO can be produced from polyamine (PA) through PA oxidase [7]. Cytochrome oxidase is also involved in the NO production.
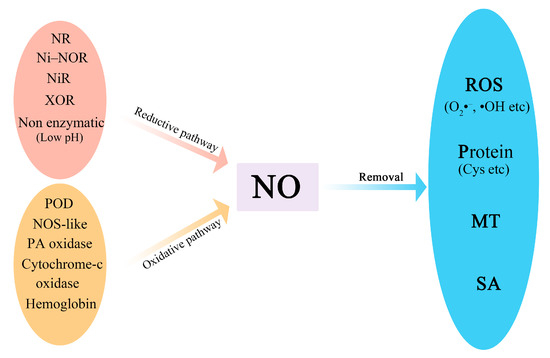
Figure 1. A schematic model of NO synthesis and removal. NO is synthesized via the reductive pathway and the oxidative pathway. The enzymes for the reductive pathway include NR, Ni–NOR, NiR, and XOR. Non-enzymatic nitrite reduction occurs spontaneously under low pH. POD, NOS-like, PA oxidase, cytochrome-c oxidase, and hemoglobin are involved in the oxidative pathway. NO can be removed by reaction with ROS, proteins, MT, and salicylic acid (SA). NR: nitrate reductase; Ni–NOR: nitrite–NO reductase; NiR: nitrate reductase; XOR: xanthine oxidoreductase; POD: peroxidase; PA: polyamine; MT: melatonin; SA: salicylic acid.
NO production has been shown to be associated with the electron transport chain in chloroplasts and mitochondria [12][13][14][12,13,14]. Under O2 deficiency, reductive NO synthesis is achieved via NO2− reduction mediated by cytochrome-c oxidase (complex IV) of the electron transport chain in mitochondria [13]. In addition, the transport of electrons to O2 is inhibited at complex IV, and the consumption of O2 is reduced. In isolated chloroplasts, NO generation has been documented and NOS-like protein appears to be involved in this process; in return, the chlorophyll biosynthesis and chloroplast differentiation can be stimulated by the increase in iron availability mediated by NO [14][15][14,15].
NO is removed when it interacts with superoxide anion (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), proteins (e.g., cysteine and tyrosine), or small signaling molecules (e.g., melatonin (MT) and salicylic acid (SA)) by oxidation reactions or S-nitrosylation. Many NO derivatives (peroxynitrite (ONOO–), S–nitrosothiols (SNO), S–nitrosoglutathione (GSNO), N–nitrosomelatonin (NOMela), dinitrogen tri-oxides, dinitrogen tetra-oxides, nitroxyl (NO–), and nitrosonium ions) are produced and involved in the regulation of plant development and stress responses [6][16][6,16]. Among these donors, SNO, GSNO, and NOMela are the main long-distance transport molecules in the NO signaling pathway.
2. Effects of NO on Fruit Ripening
The commodity value and shelf life of fruits and vegetables are greatly affected by their degree of ripeness. Short-term exposure to low concentrations of NO or its donor compounds has been shown to extend the postharvest life of various fresh fruits and vegetables. The reason might be through inhibiting ethylene (ETH) synthesis through the formation of the ternary stable complex ACO–NO–ACC or directly reducing the activity of ACS and ACO (1); inhibiting the expression of genes involved in the ETH signaling pathway (2); and affecting lignin accumulation (3). However, the inhibition of the maturation and senescence by NO depends on its concentration and specific species of fruits and vegetables (Table 1). In some species, co-treatment with other substances (e.g., 1-MCP or H2S) is more effective than treatment with NO alone (Table 1).
Table 1.
The effects of NO treatment on postharvest fruit ripening.
| Fruits | Best Treatment | Effects | References |
|---|---|---|---|
| Blueberry (Blue Cuinex, Blue Chip and Misty) |
Blue Cuinex: 1 μL L−1 1-MCP + 1 mM GSNO Misty: 1 μL L−1 1-MCP Blue Chip: Not affected by treatment. |
Maintained higher firmness, malic acid, citric acid, ascorbic acid, and glutathione contents for 14 d at 4 °C. | [17] |
| Tomato (Elpida) | 1 mM GSNO + 0.5 μL L−1 1-MCP | Delayed fruit softening, reduced the ETH synthesis significantly. | [18] |
| Red raspberry (Rubus idaeus L.) |
15 μM NO solution for 2 min (immersed in) | Reduced ETH production, respiratory intensity, ROS contents and increased the contents of flavonoids, anthocyanin, rutin, influenced metabolism of sugars. | [19] |
| Sweet pepper | 160 μM (5 ppm) NO gas for 1 h | Delayed the ripening of fruit, decreased lipid peroxidation, and increased antioxidant capacity, ascorbate content. | [20][21][22][20,21,22] |
| Banana (Brazil) | 5 mM SNP solution | Reduced ETH production, inhibited degreening of the peel, and delayed softening of the pulp. Inhibited the activity of ACO. | [23] |
| Papaya (Sui you 2) | 60 mL L−1 NO fumigated for 3 h | Suppressed ETH formation and respiratory rate (CO2 levels), reduced weight loss, maintained firmness, and delayed changes in peel color and soluble solid contents during 20 d of storage. | [24] |
| Wax apple (Syzygium samarangense) | 10 μL L−1 NO fumigated for 2 h | Lower rate of weight loss, a softening index, and loss of firmness during storage. Decreased total lignin content. | [25] |
| Tomato (Lichun) | 0.1 mM L-NAME solutions for 0.5 min | Decreased endogenous ETH release and delayed the breaker stage of fruits. | [26] |
| Peach (Dahong) | 15 μL L−1 NO + 20 μL L−1 H2S fumigated for 20 min | Inhibited ripening of peach fruits, reduced softening related enzymes activities, ETH production, ACC content, ACC synthase, and oxidase activities. | [27] |
| Water bamboo shoots (Zizania latifolia) | 30 μL L−1 NO fumigated for 4 h | Suppressed the softening and lignification effectively. | [28] |
NO can inhibit ETH synthesis during the postharvest storage of several fruits, such as mango [29], apple [30], tomato [18][26][18,26], peach [27], banana [23], strawberry [31], Chinese bayberry [32], and red raspberry [19]. The precursor of ETH is S–adenosyl methionine (SAM), which is catalyzed into amino cyclopropane carboxylic acid (ACC) by ACC synthase (ACS) and further oxidized into ETH by ACC oxygenase (ACO) [33]. NO inhibits the activity of ACS and ACO and the expression of the genes encoding these enzymes, which further affects the production of ETH and the accumulation of ACC [34][35][34,35]. Moreover, NO and ACO can be chelated by ACC to produce the ternary stable complex ACO–NO–ACC, which inhibits the ETH signaling [34][36][34,36]. NO also regulates the ETH signaling pathway by inhibiting the expression of ETH perception genes (ETH3 and ETR4) and ETH signaling pathway-related genes (EIN3-binding F-box, constitutive triple response 1, and sub-class E ethylene response factors) during the breaker stage in tomato [35]. These results indicate that NO negatively affects ETH synthesis and signaling (Figure 2).
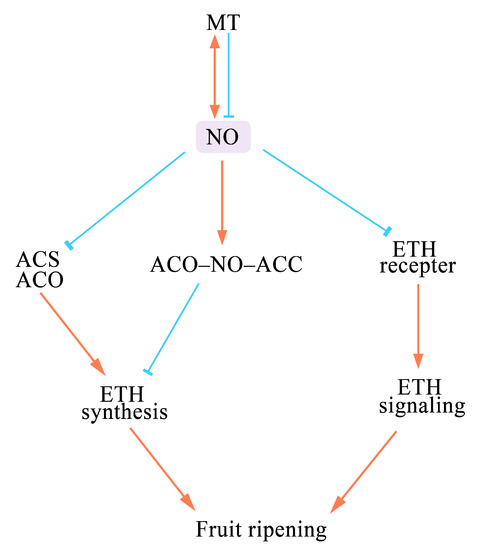
Figure 2. A schematic model of how NO affects the senescence of fruits. (“↓” indicates promote, and “┴” indicates inhibit). NO affects fruit ripening and senescence by altering ETH synthesis and signaling. It can down-regulate the activities of the ETH synthesis-related enzymes ACS and ACO to reduce ETH production. NO can also form a stable ternary ACO–NO–ACC complex to antagonize ETH formation. NO inhibits the ETH signaling pathway by down-regulating the expression of ETH perception genes. MT acts upstream of NO and can induce or inhibit NO production to regulate the senescence of fruits and vegetables. NO also induces the synthesis of MT in plants.
Interestingly, NO inhibitor treatment has also been shown to delay the ripening of tomato fruit, which is consistent with the effect of NO treatment. Thus, the mechanism by which NO inhibitor regulates ETH through its effect on NO in green mature tomato fruit differs. Treatment with the NO synthase inhibitor L–nitro–arginine methyl ester (L–NAME) in green mature tomato fruit inhibits ETH biosynthesis, which might be explained by the delay or reduction in the expression of the calcium-dependent protein kinase (CDPK) and mitogen-activated protein kinase (MAPK) genes SlCDPK1/2 and SlMAPK1/2/3 [26]. It remains unclear whether other synthetic inhibitors have the same effect.
The accumulation of lignin also affects the quality of fruits and vegetables during storage [28][37][28,37]. NO can regulate lignin synthesis by affecting the expression of genes encoding enzymes involved in lignin production, such as cinnamyl alcohol dehydrogenases and caffeoyl–CoA O–methyltransferase [37][38][37,38]. The rapid loss of tenderness in bamboo shoots (Phyllostachys violascens) mostly stems from lignification, treated with SNP could decrease the activities of phenylalanine ammonia lyase (PAL), polyphenol oxidase (PPO), and POD and lignin accumulation were also significantly reduced [39]. Moreover, NO treatment could also delay the cellulose formation and maintain the content of ascorbic acid, soluble protein, and chlorophyll in green asparagus [40]. For wax apple, after treatment with NO, the weight loss, loss of flesh firmness, and total lignin content were significantly reduced [25]. In contrast, SNP treatment may improve disease resistance by inducing lignin accumulation and enhancing the activity of PAL or POD during the storage of postharvest “Tainong” mango, kiwifruit, and wounded muskmelon [38][41][42][38,41,42]. In context, these results indicate that NO could increase or decrease lignin accumulation which may depend on the fruit and vegetable species and the biological process (ripening, senescence, or biotic stress).
