Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Pradeep R. Varadwaj and Version 2 by Rita Xu.
The pnictogen bond, a somewhat overlooked supramolecular chemical synthon known since the middle of the last century, is one of the promising types of non-covalent interactions yet to be fully understood by recognizing and exploiting its properties for the rational design of novel functional materials. Its bonding modes, energy profiles, vibrational structures and charge density topologies, among others, have yet to be comprehensively delineated, both theoretically and experimentally.
- pnictogen bonding
- nitrogen as pnictogen bond donor
- intermolecular geometries and directionality
- Halide perovskites
- Crystallography
- Energy materials
1. Introduction
Atomic nitrogen, N, the first member of the pnictogen family, is one of the major components of Earth’s atmosphere. Its molecular analogue, N2, is a crucial constituent in feedstocks leading to the production of fertilizers and mining explosives, and high-density materials [1][2][3][4][5][1,2,3,4,5]. It is likely to play an ever more important role in the economy of the future as the era of the ammonia economy and “green ammonia” approaches [4][6][7][8][9][4,6,7,8,9]. Nitrogen in numerous molecules has been recognized widely as a Lewis base, and thus can serve as an electron density donor D for the formation of various types of intermolecular interactions, including hydrogen bonds (HBs), tetrel bonds (TrBs), pnictogen bonds (PnBs), chalcogen bonds (ChBs) and halogen bonds (XBs), among others (Scheme 1a).
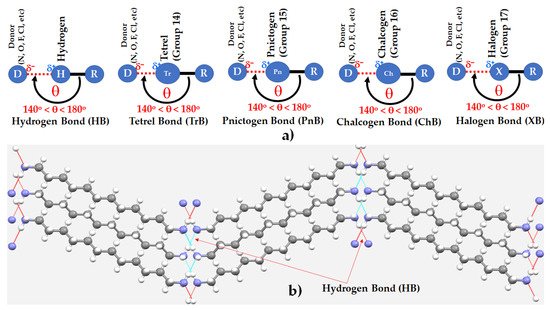
Scheme 1. (a) Schematic representation of five different types of Type-II (140° < θ < 180°) non-covalent interactions formed by covalently bonded hydrogen, tetrel, pnictogen, chalcogen, and halogen atoms in molecular entities, where R is the remainder part of the molecular entity and θ is the angular approach of the electrophile centered on the H/Tr/Ch/Pn/X atoms. (b) A ball-and-stick model of dodecane-1,12-diamine (H2N-(CH2)12-NH2), in which the ammine moiety acts both as a hydrogen bonded acceptor and a hydrogen bond donor (CSD ref: UJONUD [10][11]). The H···N hydrogen bonds in b) are depicted as dotted lines in green and red, with the latter are hanging contacts. Color codes: N—blue; C—gray; H—white.
In the crystalline phase, N2 molecules bond to each other through π···N(lone-pair) interactions, forming a variety of polymorphs referred to as the α-, β-, γ-, δ-, and ε-phases. The occurrence of these non-covalent interactions is unsurprising, given that the bonding region in N2 carries a positive potential, and the electron density accumulates along and around the extension of the N≡N triple bond [11][10].
N forms single, double and triple bonds, and, given its significant electronegativity (χPauling = 3.04; χAllen = 3.066), often carries a negative charge and its ability to act as a Lewis base is well known. Ammines can act both as hydrogen bond donors and acceptors. Scheme 1b, for instance, shows the way the nitrogen in dodecane-1,12-diamine bonds non-covalently with its neighbors through hydrogen bonds (r(H···N) = 2.233 Å, ∠N–H···N = 170.9°), resulting in the formation of a crystal lattice [10][11]. N in NH3 and ammines is a ligand in thousands of transition metal complexes, and the coordination of NH3 and its derivatives by metal ions has been extensively studied for many years, and often used to explore trends in stability constants of the transition metal ions (for example [12][13][14][15][16][17][18][19][20][21][12,13,14,15,16,17,18,19,20,21]).
There are many ammonium, diammonium and their derivatives found in solid state structures, including those that may be suitable in the development of photovoltaic and optoelectronic materials (Scheme 2). The presence of cations such as these, often referred to as spacers, or additives, stabilizes the inorganic frameworks, and hence plays a structure-determining role during the synthesis of organic-inorganic hybrid materials and is responsible for their functionality. Lower-dimensional organic-inorganic hybrid metal trihalide perovskite semiconductors are prominent examples. In these, bifunctional organoammonium cations X(CH2)2NH3+ (X = OH, Cl, Br, I, CN) control the in- and out-of-plane distortions of X(CH2)2NH3)2PbI4 perovskites [22]. The family of ⟨100⟩-oriented layered perovskites with general formula (RNH3)2An–1BnX3n+1 are obtained by taking n layers along the ⟨100⟩ direction of the parent structure [22]. A search for the fragment “–CH2–CH2–CH2–NH3” in the Cambridge Structural Database (CSD [23][24][23,24]) led to 3656 hits; that for the fragment “–X–C–NH3” or “X–CH2–NH3” (X = any atom) led to 16,834 and 9509 hits, respectively, illustrating the wide occurrence of structures featuring an RNH3+ entity. Although a few studies have discussed the importance of pnictogen bonding in the metal trihalide perovskite semiconductors (for example [25][26][25,26]), in addition to other non-covalent interactions such as hydrogen bonding and tetrel bonding [27][28][29][27,28,29], theour exploration of the geometry of a variety of crystal structures suggests that the occurrence of nitrogen-centered pnictogen bonding in these systems is very common and that it plays an important role in their structural integrity.
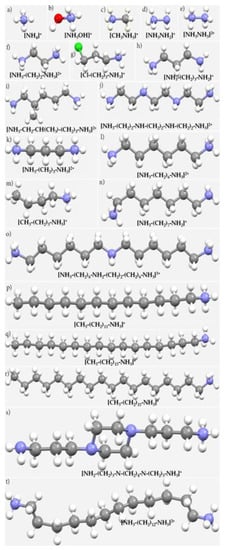
Scheme 2. Some examples of ammines, diammines, and their derivatives for in crystal structures in the CSD. These include: (a) ammonium; (b) hydroxylammonium; (c) methylammonium, (d) hydrazinium; (e) diazanediium; (f) ethylenediammonium; (g) 3-chloropropan-1-aminium; (h) 3-aminopropan-1-aminium; (i) 2-methylpentane-1,5-bis(ammonium); (j) N,N’-bis(2-ammonioethyl)ethylene-1,2-diamine; (k) propane-1,3-diaminium; (l) 1,6-hexanediammonium; (m) n-butylammonium; (n) 7-aminoheptylazanium; (o) bis(hexamethylene)triammonium; (p) dodecan-1-aminium; (q) n-hexadecylammonium; (r) octadecan-1-aminium; (s) 3-(4-(3-aminopropyl)piperazin-1-yl)propan-1-aminium; (t) dodecane-1,12-diammonium.
This overview is therefore focused on exploring representative crystal structures deposited in the CSD and the Inorganic Chemistry Structural Database (ICSD) [30][31][30,31] in which covalently bound positively-charged nitrogen contributes in part to their structural stability through inter- or intra-molecular non-covalent interactions. In many cases, rwesearchers attribute the attraction between N and the negative site as a charge-assisted nitrogen-centered pnictogen bond (or simply, nitrogen bond [11][10]). This could be either a σ-hole centered or a π-hole centered nitrogen bonding interaction. A σ-hole on an atom A is recognized along the extension of the R–A covalent bond, and is deficient in electron density, where R is the remainder part of the molecular entity [32][33][32,33]. A π-hole can be recognized on an atom (for example, N in NO3−), or on a molecular fragment (for example, the central portion of the C≡C bond in acetylene), or at the center of a delocalized system (such as an arene moiety) that has the ability to accept electron density from a lone-pair on a Lewis base (such as O in H2O and N in NH3). Examples of this have been discussed elsewhere [11][34][35][36][10,34,35,36]. TheOur survey included only single crystals in the CSD and ICSD that were free of errors and distortions, and that had an R-factor ≤ 0.1. A statistical analysis was performed using the geometric data obtained from this survey, which is presented before the conclusions section.
The nitrogen bond, or a covalently bound nitrogen-centered pnictogen bond, in chemical systems occurs when there is evidence of a net attractive interaction between the electrophilic region associated with a covalently or coordinately bound nitrogen atom in a molecular entity and a nucleophile in another, or the same, molecular entity. It is the first member of the family of pnictogen bonds formed by the first atom of the pnictogen family, Group 15, of the periodic table, and is an inter- or intra-molecular non-covalent interaction [11][10]. The possible occurrence of pnictogen bonds in many crystal lattices, formed by covalently bound nitrogen [11][33][10,33], phosphorous [33][35][33,35], arsenic [33][34][33,34], antimony [36] and bismuth [37], has already been discussed recently.
Four specific features were considered when identifying nitrogen bonding in the illustrative crystal systems chosen in this overview: (1) application of the concept of “less than the sum of the van der Waals (vdW) radii” [38][39][40][41][38,39,40,41]; (2) the directionality of the putative interaction [42][43][42,43]; (3) the positive nature of the electrostatic potential on the surface of N in molecular entities and its engagement with a negative site [11][10]; and (4) the existence of promolecular charge density-based isosurface volumes between the bonded atomic basins [44][45][44,45]. While theour discussion is largely focused on the utilization of 1 and 2 in identifying pnictogen bonding in the illustrative crystal systems, the latter two properties were computed for some chosen systems to confirm the occurrence of such interactions between molecular entities that play any appreciable role in the overall stability of the crystal lattice, and hence in the functionality of these materials. Application of the four features above has been informative in rationalizing inter- and intra-molecular interactions of various kinds [11][34][35][36][38][42][10,34,35,36,38,42] in a variety of chemical systems, and hence further demonstration is unnecessary. RWesearchers note that the appearance of N-centered pnictogen bonding in the illustrative crystals is usually accompanied by hydrogen bonds. In the majority of the crystals explored, researcherswe found that these are stronger than the nitrogen bonds; it is therefore reasonable to conclude that they are usually the cause, and that the emergence of nitrogen bonding is probably an effect.
2. Pnictogen Bonding in 1D (One-Dimensional) Perovskite Systems
Many low-dimensional halide-based organic-inorganic hybrid perovskite compounds are known [46][47][48][49][149,150,151,152]. They sometimes adopt a step-like (SL-type) structure which is effectively a 1D quantum wire with chains of corner-sharing octahedra “insulated” by blocks of face-sharing octahedra [47][150]. For instance, among others, Hoffman et al. [47][150] have reported a new series of 1D SL-type structures with the general formula (PA)2m+4(MA)m−2Pb2m+1I7m+4 (m = 2, 3, 4), where the PbI6 octahedra connect in a corner- and face-sharing motif that exhibit resistivity trends that are dominated by ionic transport and no photoresponse. Yuan and coworkers [50][153] have reported the synthesis, crystal structure and photophysical properties of a 1D organic lead bromide perovskite, C4N2H14PbBr4. It consists of edge-sharing octahedral lead bromide chains [PbBr42−]n that are surrounded by the C4N2H142+ organic cations to form the bulk assembly of core-shell quantum wires. The 1D structure has enabled the authors to demonstrate the presence of strong quantum confinement with the formation of self-trapped excited states that give efficient bluish white-light emissions with photoluminescence quantum efficiencies of approximately 20% for the bulk single crystals and 12% for the microscale crystals. Shown in Figure 1 are a few illustrative 1D crystal structures that are stabilized by the joint involvement of hydrogen- and pnictogen-bonding interactions between the organic and inorganic frameworks.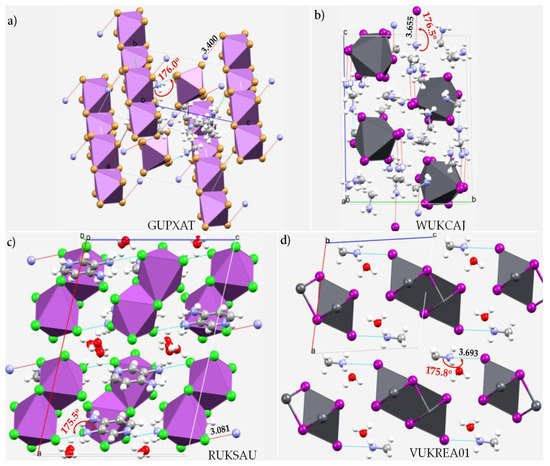
Figure 1. Ball-and-stick and polyhedral models of selected 1D structure. (a) bis(N1,N1-diethylethane-1,2-bis(aminium)) bis(μ-bromo)-octabromo-di-antimony (C6H18N22+)2(Br10Sb24−) [51][154]; (b) pentakis(methylammonium) tris(μ-iodo)-hexakis(iodo)-di-lead bis(methylamine) (CH6N+)5(I9Pb25−),2(CH5N) [52][155]; (c) bis(3-ammoniumyl-4-amino-pyridin-1-ium) bis(μ-chloro)-octachloro-di-bismuth dehydrate (C5H9N32+)2(Bi2Cl104−)2(H2O) [53][54][156,157]; (d) catena-[methylammonium bis(μ-iodo)-iodo-lead monohydrate (CH6N+)(I3Pb−)n,(H2O) [55][158]. Intermolecular contacts between bonded atomic basins are illustrated as dotted lines in cyan and hanging contacts in red. Selected bond distances and bond angles associated with the nitrogen-centered pnictogen bonds are in Å and degrees, respectively.
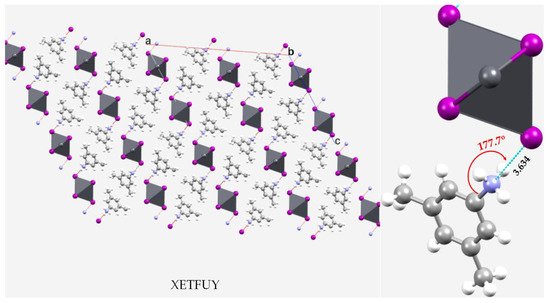
Figure 2. Ball-and-stick and polyhedral model of the 2 × 2 × 2 cell of the crystal of catena-(bis(3,5-dimethylanilinium) bis(μ2-iodo)-diiodo-lead(II)) [(C8H12N+)2n, (I4Pb2−)] [56][159]. Intermolecular contacts between bonded atomic basins are illustrated as dotted lines in cyan and hanging contacts in red. Selected bond distance and bond angle associated with the nitrogen-centered pnictogen bond is in Å and degrees, respectively. The CSD reference is shown in uppercase letters.
